

Background: B cells play a pivotal role in the pathogenesis of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV), as evidenced by the efficacy of B-cell depleting therapy with the monoclonal anti-CD20 agent, rituximab (RTX), in multiple randomized controlled trials [1–4]. RTX is now the standard of care for both induction and maintenance of remission in AAV, as endorsed by the latest guidelines [5,6]. Achieving sustained remission is crucial to minimize the risk of relapses, which can lead to long-term organ damage, both from disease activity itself and from prolonged immunosuppressive therapy. However, reliable predictors of relapse remain elusive. CD19+ B-cell quantification is recommended as part of disease activity monitoring in AAV, yet its prognostic significance is still debated.
Objectives: This study aimed to determine the prevalence of incomplete B-cell depletion during RTX maintenance therapy in AAV patients and to evaluate its impact on the risk of relapse and disease management.
Methods: We conducted a retrospective, single-center cohort study at Cochin Hospital, including patients who fulfilled the 2022 ACR/EULAR classification criteria for AAV and had received at least two years of RTX maintenance therapy (fixed-dose, 500 mg every 6 months). Clinical, biological, and treatment data were collected and analyzed.
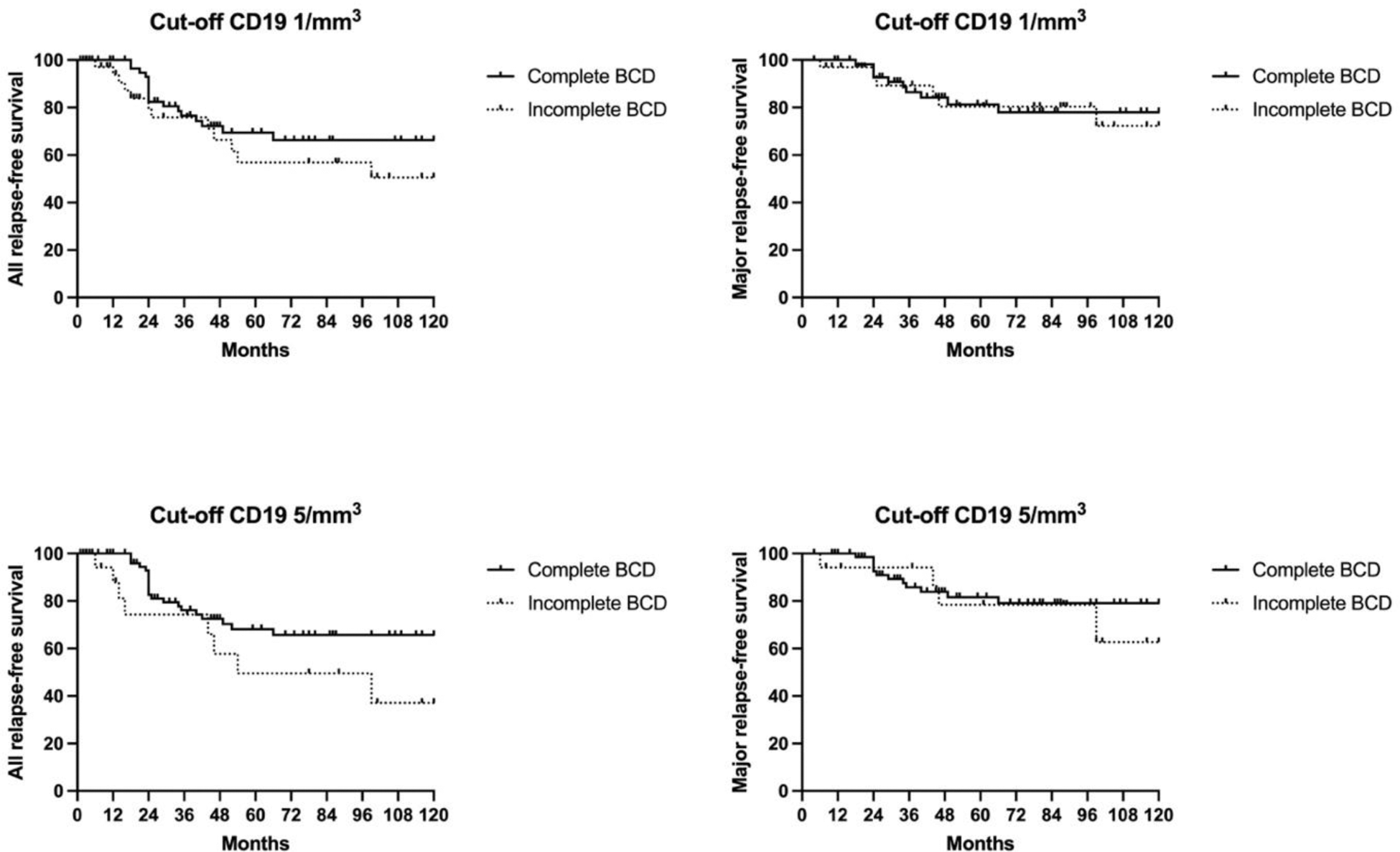
Results: Among 202 patients screened, 175 were included (Table 1). Induction therapy prior to RTX maintenance comprised RTX (375 mg/m²/week in 65.7%, or 1 g two weeks apart in 21.1%) or cyclophosphamide (13.7%). The median duration of RTX maintenance was 19 months (IQR 19–37). At initiation of RTX maintenance (Month 0), incomplete B-cell depletion (CD19+ ≥1/µL) was observed in 31.4% of patients. The proportion of incomplete B-cell depletion remained stable during follow-up: 28.6% at Month 6, 32.6% at Month 12, and 33.1% at Month 18. During RTX maintenance, 9.7% of patients experienced relapses (12 minor, 9 major), with incomplete B-cell depletion in 47.4% and complete depletion in 52.6% of relapsing cases. After 18 months, 37.7% of patients continued RTX maintenance for additional 24 (IQR 14-25) months, and 62.3% of patients discontinued RTX maintenance, with a follow-up of 58 months (IQR 32–81) after the last RTX infusion. Among the latter, 27.5% experienced relapses post-RTX discontinuation (13 minor, 17 major). All relapse and major relapse-free survival were comparable between patients with incomplete and complete B-cell depletion during RTX maintenance, regardless of the CD19+ threshold used (1/µL or 5/µL) (Figure 1).
Conclusion: Our data suggest that approximately one third of AAV patients exhibit incomplete B-cell depletion during RTX maintenance therapy. However, this was not associated with an increased risk of relapse, suggesting that achieving complete B-cell depletion may not be mandatory for effective disease control during maintenance.
REFERENCES: [1] Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med . 2014;371:1771–80. DOI: 10.1056/NEJMOA1404231.
[2] JH S, PA M, R S, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med . 2010;363:168. DOI: 10.1056/NEJMOA0909905.
[3] Jones RB, Cohen Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med . 2010;363:211–20. DOI: 10.1056/NEJMOA0909169.
[4] Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med . 2013;369:417–27. DOI: 10.1056/NEJMOA1213277.
[5] Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis . 2024;83:30–47. DOI: 10.1136/ARD-2022-223764.
[6] Floege J, Jayne DRW, Sanders JSF, et al. KDIGO 2024 Clinical Practice Guideline for the Management of Antineutrophil Cytoplasmic Antibody (ANCA)–Associated Vasculitis. Kidney Int . 2024;105:S71–116. DOI: 10.1016/j.kint.2023.10.008.
Baseline clinical features of the study cohort
| N=175 | |
|---|---|
| Median (IQR) age at diagnosis | 53 (36-66) |
| Female | 102 (58,3%) |
| GPA | 141 (80,6%) |
| MPA | 34 (19,4%) |
| PR3-ANCA | 101 (57,7%) |
| MPO-ANCA | 55 (31,4%) |
| newly diagnosed disease | 91 (52%) |
| relapsing disease | 84 (48%) |
| ENT involvement | 124 (70,8%) |
| Pulmonary involvement | 110 (62,8%) |
| Renal involvement | 91 (52%) |
| Nervous system involvement | 34 (19,4%) |
| Ocular involvement | 54 (30,8%) |
| GI involvement | 11 (6,3%) |
| Cardiac involvement | 12 (6,8%) |
| median (IQR) BVAS | 9 (6-15) |
| median (IQR) CD19/µl | 109 (30-219) |
| RTX induction regimen | 152 (86,8%) |
| CYC induction regimen | 24 (13,7%) |
| FFS ≥ 1 | 89 (50,8%) |
Data are reported as n (%) or mean (SD).
ANCA= antineutrophil cytoplasmic antibodies; BVAS= Birmingham Vasculitis Activity Score; CYC= cyclophosphamide; ENT=ear, nose, throat; FFS= five factor score; GI= gastrointestinal; GPA= granulomatosis with polyangiitis; MPA= microscopic polyangiitis; MPO= myeloperoxidase; PR3= proteinase 3; RTX= rituximab.
Kaplan-Meier curves illustrating all relapse-free survival and major relapse-free survival according to B-cell depletion status during rituximab (RTX) maintenance therapy in ANCA-associated vasculitis patients. (Upper left) All relapse-free survival based on a CD19+ B-cell threshold of 1/mm³; (Upper right) Major relapse-free survival based on a CD19+ B-cell threshold of 1/mm³; (Lower left) All relapse-free survival based on a CD19+ B-cell threshold of 5/mm³; (Lower right) Major relapse-free survival based on a CD19+ B-cell threshold of 5/mm³. Solid lines represent patients with complete B-cell depletion, while dashed lines indicate those with incomplete B-cell depletion.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (