

Background: Failure of standard treatments in psoriatic arthritis (PsA) leaves many patients functionally impaired with chronic symptoms. These patients may be classified as treatment-refractory or ‘difficult-to-treat’ (D2T). Currently, no established definition of D2T PsA exists.
Objectives: To explore the impact of increasingly strict definitions on the prevalence of D2T PsA and to describe characteristics of the patients fulfilling the applied D2T definitions.
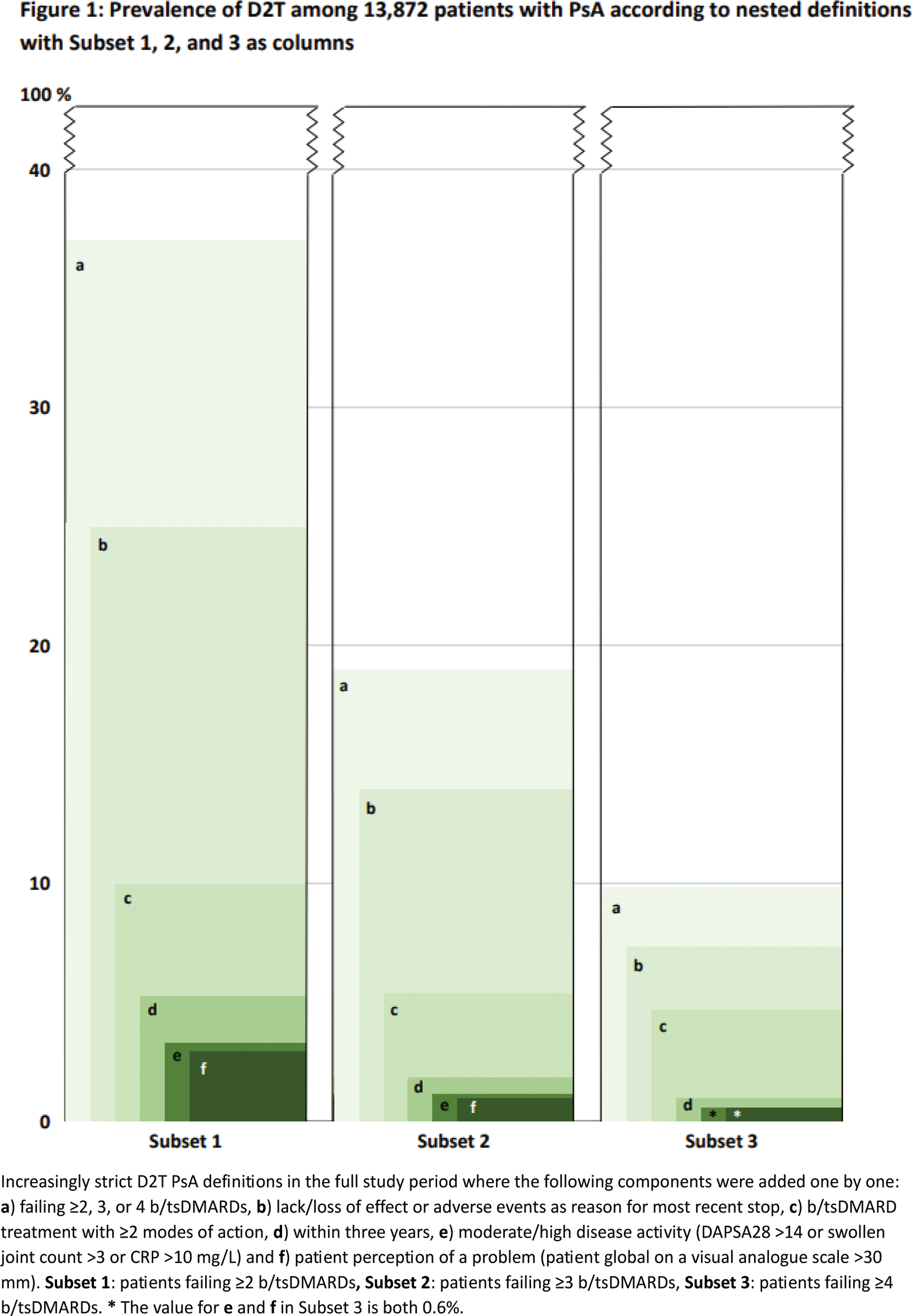
Methods: Observational cohort study including patients initiating a first-ever biologic or targeted synthetic disease-modifying anti-rheumatic drug (b/tsDMARD) from 1999 to 2022 in the Nordic biologic registries (Denmark DANBIO-DRQ/Sweden SRQ/Finland ROB-FIN/Norway NOR-DMARD/Iceland ICEBIO) [1] with >2 years of follow-up after treatment start (=baseline). We explored a range of register-based D2T definitions inspired by the corresponding pre-existing definitions in RA [2] and axSpA [3]. Thus, the prevalence of patients with D2T were explored in three nested cohorts defined as a ) treatment failure of ≥2, ≥3, or ≥4 b/tsDMARDs during follow-up in the registry. In each of these three subsets, defined by a ) (here termed subset 1, 2, and 3, respectively), the following components were then added one by one regarding the b/tsDMARD treatment history: b ) lack/loss of effect or adverse event as reason for stopping the most recent b/tsDMARD; c ) failure of ≥2 different modes of action; d ) all failures occurred within three years from baseline. Finally, based on data from the most recent visit during treatment with the most recent b/tsDMARD, two further components were sequentially added to illustrate suboptimal disease control: e ) moderate/high disease activity (defined as Disease Activity index in PSoriatic Arthritis based on 28 joint-counts (DAPSA28) >14, or swollen joint count >3 (of 28), or CRP >10 mg/L); f ) patient perception of a problem (patient global visual analogue scale >30 mm). Patient characteristics at baseline were described in the full cohort and for subset 1, 2 and 3, respectively. Corresponding characteristics limited to patients initiating a first b/tsDMARD after 2015 were also presented to illustrate the more recent treat-to-target era.
Results: Among 17,456 patients with PsA identified in the registries, a total of 13,872 patients with at least two years of follow-up were included (52% females; mean age 48 years; disease duration 5.6 years; DAPSA28: 33 (Table 1)). Of these, 37% met definition a by failing ≥2 b/tsDMARDs (subset 1), 19% failed ≥3 (subset 2), and 10% failed ≥4 b/tsDMARDs (subset 3). The mean time from the first b/tsDMARD start until the failure of the second, third, and fourth b/tsDMARDs was 5, 6, and 7 years, respectively. When all components ( b to f ) were added, the prevalence of patients fulfilling definitions dropped to 3%, 1%, and 0.6%, respectively (Figure 1). Patients failing ≥2, ≥3, and ≥4 b/tsDMARDs were more often females (61%, 64%, and 67%, respectively).In the patient subset initiating the first b/tsDMARD after 2015, the mean time from start of first b/tsDMARD until the failure of the second, third, and fourth b/tsDMARDs was 3, 3, and 4 years, respectively. In the period after 2015, the time from diagnosis until start of the first b/tsDMARD was numerically slightly shorter, and the proportion of females failing ≥2, ≥3, and ≥4 b/tsDMARDs was larger compared to those initiating therapy prior to 2015 (data not shown).
Conclusion: Our findings indicate that multiple b/tsDMARD failures in PsA, indicating D2T disease, are common, particularly among females. Depending on the definition of D2T PSA, the proportions varied widely, ranging from 0.6% to 37%, illustrating the impact of adding further and more strict components. These insights are valuable for the ongoing process of developing a universally accepted definition of D2T PsA.
REFERENCES: [1] Glintborg B, et al. SJR. 2018. DOI: 10.1080/03009742.2018.1444199.
[2] Nagy G, et al. ARD. 2021. DOI: 10.1136/annrheumdis-2020-217344.
[3] Poddubnyy D, et al. Abstract ACR 2024 (No 0819).
Table 1. Patients with PsA failing ≥2, ≥3, or ≥4 b/tsDMARDs (definition a), with characteristics at baseline and at stop of the latest treatment.
Full cohort and the subset of patients with start of first b/tsDMARD after 2015.
| Full study period (1999-2022)
| Subset of patients after 2015*
|
|||||||
|---|---|---|---|---|---|---|---|---|
| All patients | Failing ≥2
| Failing ≥3
| Failing ≥4
| All patients | Failing ≥2
| Failing ≥3
| Failing ≥4
|
|
| N (%**) | 13872 | 5079 (37) | 2584 (19) | 1365 (10) | 5909 | 1523 (26) | 639 (11) | 289 (5) |
| Baseline characteristics | ||||||||
| Female, % | 52 | 61 | 64 | 67 | 53 | 65 | 69 | 70 |
| Age, years | 48 (13) | 47 (13) | 46 (13) | 46 (12) | 49 (13) | 49 (13) | 48 (13) | 47 (12) |
| Disease duration***, years | 5.6 (7.4) | 5.16 (7.0) | 5.10 (7.2) | 4.99 (7.3) | 5.2 (7.1) | 4.6 (6.4) | 4.5 (6.5) | 4.5 (6.9) |
| DAPSA28 | 34 (32) | 31 (31) | 33 (32) | 33 (30) | 32 (32) | 32 (35) | 33 (34) | 37 (39) |
| Characteristics when failing most recent b/tsDMARD | ||||||||
| VAS Global Score, 0-100 mm | - | 60 (25) | 63 (24) | 67 (23) | - | 63 (25) | 65 (24) | 70 (22) |
| Time since start of first b/tsDMARD, years | - | 4.5 (3.9) | 5.9 (4.1) | 7.0 (4.0) | - | 2.5 (1.7) | 3.1 (1.6) | 3.6 (1.6) |
| Reason for stop of latest b/tsDMARD, % | ||||||||
| -Lack/loss of effect | - | 44 | 49 | 54 | - | 43 | 47 | 51 |
| -Adverse event | - | 18 | 17 | 16 | - | 18 | 20 | 18 |
| -Other | - | 26 | 22 | 19 | - | 18 | 15 | 11 |
| -Missing | - | 12 | 12 | 11 | - | 21 | 18 | 20 |
| Having stopped all b/tsDMARDs due to lack/loss of effect or adverse events, % |
| 49 | 43 | 39 | - | 46 | 42 | 41 |
Numbers are mean (SD) unless otherwise shown. Baseline = start of first biologic or targeted synthetic disease-modifying anti-rheumatic drug (b/tsDMARD). *These patients are a subset of the patients included in the full study period, **Percentage of all patients, ***From diagnosis until first b/tsDMARD start. Missing data range from 0 to 36 %. Visual analogue scale (VAS). Disease Activity index in PSoriatic Arthritis based on 28 joint-counts (DAPSA28)
Acknowledgements: To Ulf Lindström for assistance in preparing the metadata sheet. Partly funded by grants from FOREUM, Vinnova and NordForsk. Partly funded by The Danish Rheumatism association.
Disclosure of Interests: Louise Majormoen Nielung: None declared, Daniela Di Giuseppe: None declared, Merete Lund Hetland Medac, Novartis, Pfizer, Sandoz, UCB, AbbVie/Abbott, Bristol-Myers Squibb (BMS), Eli Lilly, Merck/MSD, Novartis, Pfizer, Sandoz, UCB, Johan Askling Abbvie, BMS, Eli Lilly, Galapagos NV/Alfasigma S.p.A, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, Dan Nordström UCB, Pfizer, Pfizer, UCB, Lilly, MSD, Novartis, Pfizer, UCB, BMS, MSD, UCB, Sella Aarrestad Provan Pfizer and Boehringer Ingelheim, Boehringer Ingelheim and Novartis, Bjorn Gudbjornsson: None declared, Ólafur Pálsson: None declared, Lene Dreyer MSD, UCB, Eli Lillly, AbbVie and BMS (payment to institution, Brigitte Michelsen Novartis and Pfizer (paid to employer), Bénédicte Delcoigne: None declared, Katerina Chatzidionysiou: None declared, Johan K Wallman AbbVie, Amgen, AbbVie, Amgen, Eli Lilly, Novartis, Pfizer, Bente Glintborg AbbVie/Abbott, Bristol-Myers Squibb (BMS), Pfizer, Sandoz.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (