

Background: The Human Leukocyte Antigen (HLA) region, particularly the HLA-B and HLA-C genes, plays a crucial role in susceptibility to and the clinical phenotype of Psoriatic Arthritis (PsA).
Objectives: This study aimed to investigate the HLAs associated with the occurrence and course of acute/tender dactylitis in PsA patients.
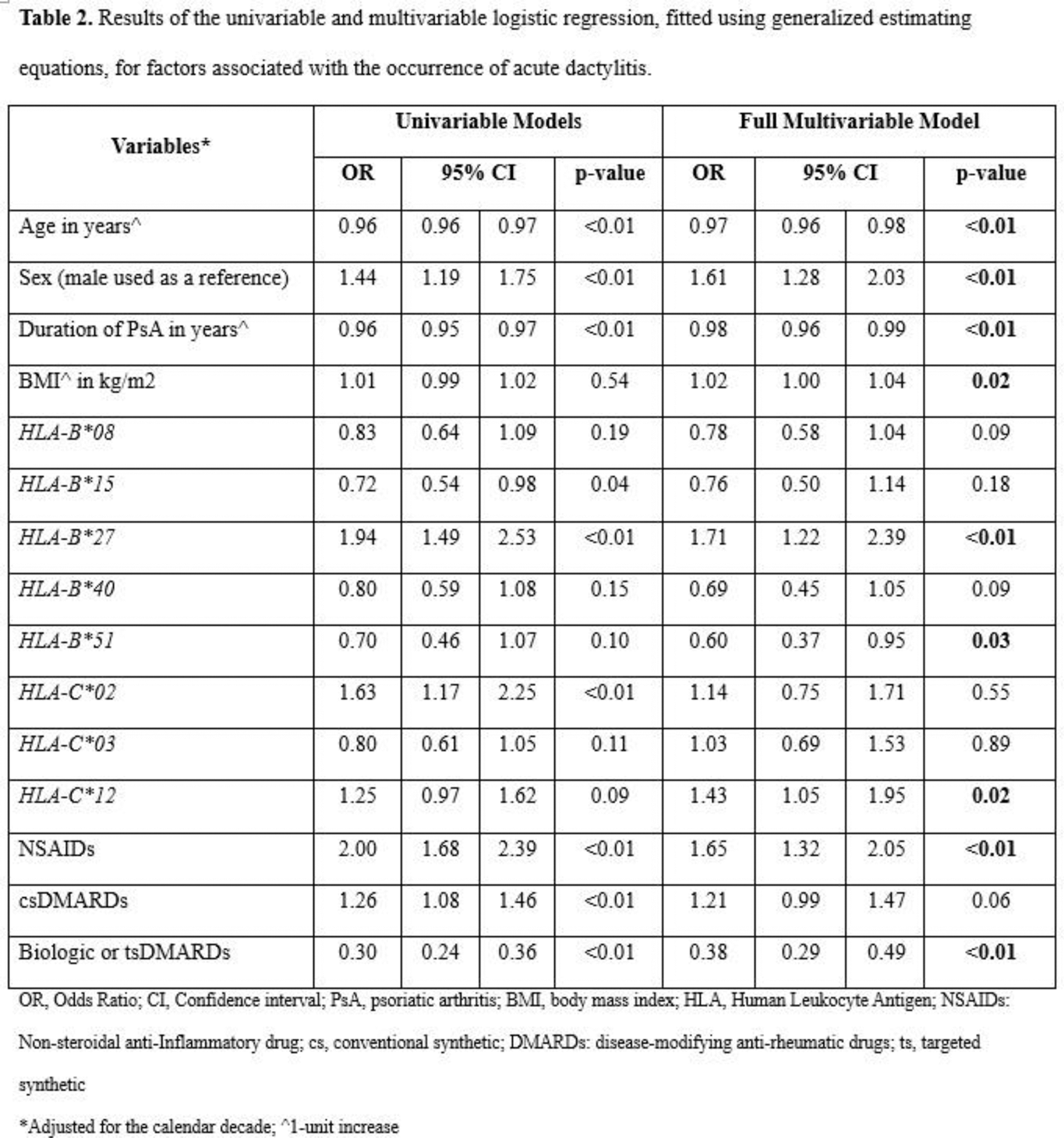
Methods: We analyzed data from a prospective observational cohort of PsA patients. We identified visits with acute dactylitis and screened each HLA-B or HLA-C allele with a prevalence >5% in our database for an association with this outcome by employing univariable logistic regression, fitted using generalized estimating equations (GEE). We selected alleles with a p-value ≤0.2 for further analysis, including HLA-B*08, HLA-B*15, HLA-B*27, HLA-B*40, HLA-B*51, HLA-C*02, HLA-C*03, and HLA-C*12 . We then performed a multivariable logistic regression, fitted using GEE and adjusted for the calendar decade (1978-1988 used as reference, 1989-1999, 2000-2010, 2011-2024), to examine factors associated with the occurrence of acute dactylitis. We used Cox regression models, adjusted for the calendar decade, to explore factors influencing the time to resolution of the first event of acute dactylitis. Lastly, we performed sensitivity analyses incorporating clinically relevant haplotypes ( HLA-B*8/C*07, HLA-B*27/C*01, HLA-B*27/C*02, HLA-B*38/C*12 , HLA-B*44/C*05, HLA-B*44/C*16 and HLA-B*57/C*06 ) instead of alleles.
Results: Among the 1216 patients included, 612 (52%) experienced at least one event of acute dactylitis over a median follow-up duration of 12.0 [IQR: 5.7–20.1] years (Table 1). Six hundred and ninety-nine (57.5%) patients were male, with a mean age at baseline (clinic entry) of 44.0 (SD 12.9) years. The proportion of patients with HLA-B*27 was 16.6%, HLA-B* 38/C* 12 was 10.9%, and 8.6% were HLA-B* 27/C*02 positive. In the multivariable GEE analysis (Table 2), adjusted for the calendar decade, we found that the presence of HLA-B*27 (OR 1.71, 95% CI 1.22–2.39) and HLA-C* 12 (OR 1.43, 95% CI 1.05–1.95) alleles was positively associated with the occurrence of acute dactylitis. In contrast, HLA-B*51 (OR 0.60, 95% CI 0.37–0.95) showed a negative association. Other clinical factors independently associated with the occurrence of acute dactylitis included younger age in years (OR 0.97, 95% CI 0.96–0.98), male sex (OR 1.61, 95% CI 1.28–2.03), shorter duration of PsA (OR 0.98, 95% CI 0.96–0.99), higher body mass index (OR 1.02, 95% CI 1.01–1.04), and the use of non-steroidal anti-inflammatory drugs (OR 1.65, 95% CI 1.32–2.05). Conversely, having been on biologic or targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs) at the time of the acute dactylitis (OR 0.38, 95% CI 0.29–0.49) showed a negative association. In the multivariable Cox regression model, adjusted for the calendar decade, we found that none of the HLA alleles was independently associated with the time to resolution of acute dactylitis. Initiating biologic or targeted synthetic DMARDs was associated with faster resolution of acute dactylitis (OR 1.67, 95% CI 1.17–2.36). In sensitivity analyses incorporating haplotypes instead of alleles, the haplotypes HLA-B* 38/C* 12 (OR 1.51, 95% CI 1.14–2.01) and HLA-B* 27/C* 02 (OR 1.69, 95% CI 1.17–2.44) were independently associated with the occurrence of acute dactylitis. None of the haplotypes showed a significant association with the time to resolution of acute dactylitis.
Conclusion: HLA-B*27 and HLA-C*12 alleles, as well as the haplotypes HLA-B*38/C* 12 and HLA-B*27/C* 02, are positively associated with the occurrence of acute PsA dactylitis. In contrast, HLA-B*51 shows a negative association. None of these HLAs are associated with the course of acute dactylitis, investigated as the time to resolution. These findings may contribute to a deeper understanding of the interplay between genetic factors and clinical outcomes in PsA.
REFERENCES: NIL.
Patient characteristics at the time of clinic entry.
| Variable | Overall
| Never had dactylitis
| Ever had dactylitis
|
|
|---|---|---|---|---|
| Age in years, mean (SD) | 44.0 (12.9) | 46.0 (13.2) | 41.9 (12.3) | |
| Sex (male), n (%) | 699 (57.5) | 332 (52.5) | 367 (62.8) | |
| Ethnicity | White, n (%) | 1033 (85.3) | 520 (82.5) | 513 (88.3) |
| Duration of PsA in years, median [IQR] | 2.9 [0.8, 8.4] | 2.8 [0.7, 8.9] | 2.9 [0.8, 8.3] | |
| BMI in kg/m2, mean (SD) | 28.8 (6.2) | 28.73 (6.45) | 28.8 (5.98) | |
| Acute dactylitis, n (%) | 338 (27.9) | - | 338 (58.0) | |
| PASI (0-72), median [IQR] | 1.8 [0.0, 4.6] | 1.8 [0.0, 4.9] | 1.4 [0.0, 4.4] | |
| DAPSA, median [IQR] | 16.8 [9.0, 29.0] | 16.6 [9.8, 28.7] | 17.0 [9.0, 29.0] | |
| Modified Steinbrocker score, median [IQR] | 2.0 [0.0, 9.0] | 0.0 [0.0, 6.0] | 2.0 [0.0, 12.0] | |
| Sacroiliitis, n (%) | 246 (23.2) | 117 (22.1) | 129 (24.3) | |
| CRP in mg/dL, mean (SD) | 13.8 (20.7) | 13.2 (20.2) | 14.8 (21.6) | |
| HLA-B*27 , n (%) | 201 (16.6) | 76 (12.1) | 125 (21.4) | |
| HLA-B*51 , n (%) | 70 (5.8) | 38 (6.0) | 32 (5.5) | |
| HLA-C*12 , n (%) | 253 (20.9) | 121 (19.2) | 132 (22.8) | |
| HLA-B*27/C*02 , n (%) | 105 (8.6) | 35 (5.5) | 70 (12.0) | |
| HLA-B*38/C*12 , n (%) | 133 (10.9) | 58 (9.2) | 75 (12.8) | |
| NSAIDs, n (%) | 819 (67.4) | 385 (60.9) | 434 (74.3) | |
| csDMARDs, n (%) | 467 (38.4) | 232 (36.7) | 235 (40.2) | |
| Biologic or tsDMARDs, n (%) | 105 (8.6) | 78 (12.3) | 27 (4.6) | |
SD, standard deviation; PsA, psoriatic arthritis; IQR, interquartile range; BMI, body mass index; PASI, Psoriasis Area and Severity Index; DAPSA, Disease Activity Index for Psoriatic Arthritis; CRP, C-reactive protein; HLA, Human Leukocyte Antigen; NSAIDs: Non-steroidal anti-Inflammatory drug; cs, conventional synthetic; DMARDs: disease-modifying anti-rheumatic drugs; ts, targeted synthetic
Acknowledgements: NIL.
Disclosure of Interests: Fadi Kharouf: None declared, Virginia Carrizo Abarza: None declared, Pankti Mehta: None declared, Shangyi Gao: None declared, Darshini Ganatra: None declared, Daniel Periera: None declared, Richard Cook: None declared, Denis Poddubnyy AbbVie, Canon, DKSH, Eli Lilly, Janssen, MSD, Medscape, Novartis, Peervoice, Pfizer, and UCB, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Janssen, Moonlake, Novartis, Pfizer, and UCB, AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Dafna D. Gladman AstraZeneca, Abbvie, Amgen, BMS, Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Pfizzer, UCB, Vinod Chandran AbbVie, BMS, Eli Lilly, Fresenius Kabi, Johnson and Johnson, Novartis, UCB, AbbVie, Eli Lilly.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (