

Background: Treatment guidelines for rheumatoid arthritis (RA) recommend initiating biologic disease-modifying anti-rheumatic drugs (bDMARDs) in patients who have inadequate response to first-line conventional synthetic DMARDs (csDMARDs) and present with poor prognostic factors. Studies indicate that a considerable proportion of patients meet these criteria and would therefore be candidates for bDMARD therapy [1]. However, in clinical practice, many patients transition to or receive an additional csDMARD instead of a bDMARD, a strategy that may prove both effective and advantageous.
Objectives: To evaluate the effectiveness and patterns of csDMARD strategies in early RA patients who failed first-line methotrexate (MTX), across European healthcare systems.
Methods: We used data from two European regions: Region Stockholm (Sweden, SW, 2008-2023), Leiden (Netherlands, NL, 2011-2024). We included all newly diagnosed RA patients by rheumatologists at each centre who had failed MTX monotherapy as their first-ever DMARD. Patients who had initiated two distinct medications within a 30-day period were designated as one combined therapy. We compared two types of changes a) switchers – patients who stopped MTX and received a new csDMARD, and b) add-on – patients who were maintained on MTX but added an additional csDMARD. Patient characteristics were analysed across treatment strategies and specific medications. Treatment effectiveness was evaluated by i) disease activity at 3, 6, and 12 months, and ii) medication survival analysis per therapeutic strategy. Differences in the latter were assessed using log-rank tests.
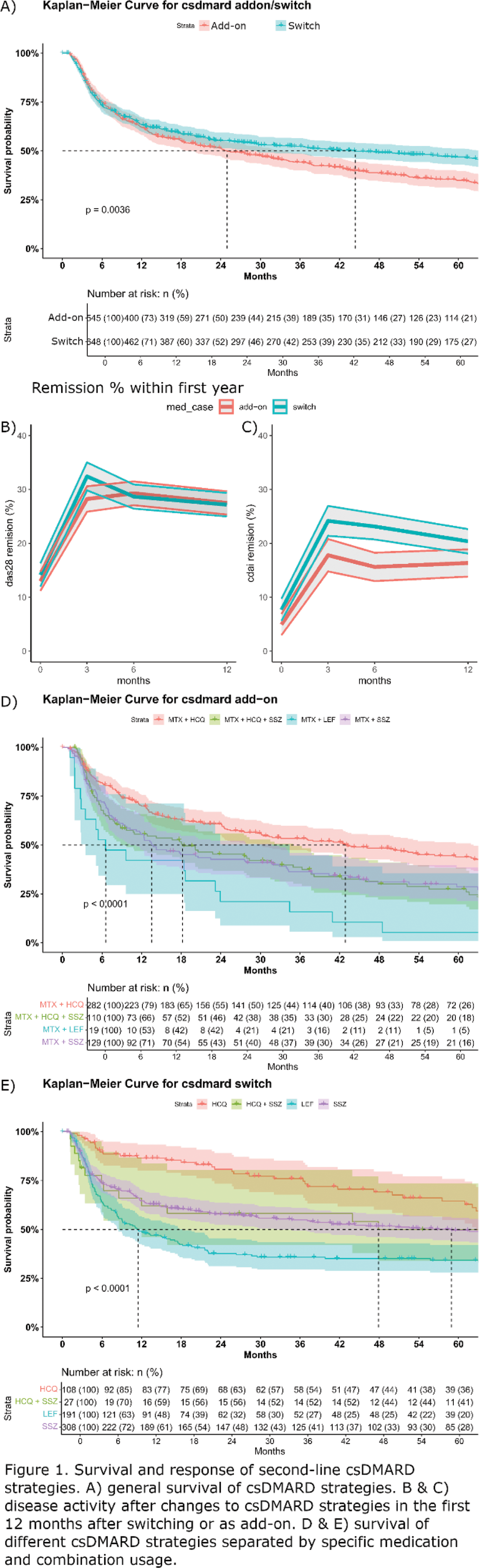
Results: Of the total 5,522 patients (3850 from SE and 1672 from NL) with early RA, 4,606 started mono-MTX and 2,198 failed mono-MTX. Of these 2,198, 1,193 (54.3%) received a csDMARD (either as add-on or switched to) as second-line treatment, 37% in the SE and 92% in the NL datasets. Switching from MTX to another csDMARD was the most common DMARD treatment strategy (54%). These were sulfasalazine (SSZ, 48%), leflunomide (LEF, 29%) and hydroxychloroquine (HCQ,17%) in the combined data (Table 1). At country level, the 2 nd line single DMARD switch differed: 43% and 10% for SSZ, 15% and 17% for LEF, 13% and 5% for HCQ for SE and NL respectively. The remaining 46% received their second csDMARD as an add-on to MTX. These were SSZ (24%), HCQ (52%), HCQ+SSZ (20%) and LEF (4%) in the combined data (Table 1). At country level the 2 nd line DMARD switch differed: 63% and 10% for SSZ, 18% and 65% for HCQ, 13% and 23% for HCQ+SSZ for SE and NL respectively. Switchers were more frequently women (76.1% vs 65.9%) and had on average a higher DAS28 score at the time of switch than those in the add-on group (4.2 vs 3.9). On average patients stopped mono-MTX and adding/switching another csDMARDs after 9 months. To longest time to change from mono-MTX was to HCQ monotherapy. Patients receiving SSZ and MTX showed similar poor prognostic factors (elevated DAS28, seropositivity) and time-to-modification intervals as those using triple therapy (SSZ/MTX/HCQ). HCQ add-on patients demonstrated comparable prognostic factors as those who switched from MTX to SSZ monotherapy. Patients receiving HCQ demonstrated the lowest DAS28 scores at the time of switching, or the lowest scores when compared to the add-on strategies. Figure 1A shows the survival of second-line csDMARD strategies were similar for the switch and add-on strategy in the first year, but deviated after the 12 months with a poor survival for the add-on strategy. The average survival of an add-on strategy is 24 months, while it is 44 months for the switchers. Figure 1B and C show the DAS28 responses were similar for the switchers and add-on strategy, a stricter CDAI remission showed a better outcome for patients who switched than the add-on strategy. Figure 1D and E revealed HCQ achieving a superior survival rate compared to all other medications, regardless of treatment strategy. SSZ+HCQ and SSZ monotherapy demonstrated similar survival curves when compared within either the switch or add-on strata. Notably, LEF demonstrated significantly lower survival rates in both switch and add-on scenarios.
Conclusion: In early RA patients who failed MTX monotherapy, switching to a different csDMARD showed better treatment survival (44 months) compared to add-on strategies (24 months). Treatment selection appeared to be influenced by disease severity, with HCQ preferred in less severe cases and showing superior survival rates. LEF demonstrated poor survival rates in both strategies. Triple therapy with HCQ added to SSZ and MTX showed no additional survival benefit compared to dual therapy.
REFERENCES: [1]
Acknowledgements: This project has received funding from Horizon Europe programme under grant agreement no. 101095052 (SQUEEZE), no. 101080711 (SPIDERR), ZonMW klinische fellow no. 40-00703-97-19069, and ZonMw Open Competitie 2021 no. 09120012110075. We thank David Steeman for his help in extracting and processing the electronic health record data.
Disclosure of Interests: Nils Steinz: None declared, Daniela Di Giuseppe: None declared, Tjardo D. Maarseveen: None declared, Claudia Anna Hana: None declared, Joe Sexton: None declared, Claudiu POPESCU: None declared, Corina Mogosan: None declared, Catalin Codreanu: None declared, Sella Aarrestad Provan: None declared, Helga Lechner-Radner: None declared, Daniela Sieghart: None declared, Johan Askling agreements between Karolinska Institutet (with JA as PI) and the listed entities, mainly for the national safety monitoring of rheumatology immunomodulators in Sweden (ARTIS): Abbvie, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi;, Rachel Knevel: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (