

Background: Previous studies on cellular immunity in rheumatoid arthritis (RA) treatment have mostly targeted synovial tissue (ST). The synovium has long been recognized as an important target organ in RA involvement joint, but it can usually be obtained during surgery and is not suitable as a routine method for disease diagnosis and evaluation of efficacy. Therefore, we have turned our attention to synovial fluid (SF), which is co-located with ST in the inflammatory environment of the joint cavity. Additionally, cytological investigations relying on traditional approaches have hampered the ability to systematically explore the damaging role of immune cells in RA at the single-cell level due to the low throughput. With the development of single-cell RNA sequencing (scRNA-seq), multiple studies have delineated the cellular transcriptional profile of different cell components of ST from RA patients [1, 2], but a complete single-cell immunoprofile of RA synovial fluid, along with its variations before and after treatment remains elusive to this day.
Objectives: This study aims to construct a single-cell atlas of RA synovial fluid, explore the dynamic immunoprofiles of cell components impacted by the treatment with adalimumab/tofacitinib, and expect to screen key cell subpopulations involved in RA pathogenesis from SF.
Methods: Six RA patients and three osteoarthritis (OA) patients were included. RA patients were randomized to receive either the TNF-α inhibitor adalimumab (n=3) or the JAK inhibitor tofacitinib (n=3) after enrollment. Knee SF from 6 RA patients before treatment (referred to as RA-BT) and 1 month after receiving treatment (referred to as RA-AT), as well as SF from 3 OA patients, was collected for scRNA-seq to map the single-cell immunoprofiles of RA synovial fluid. Based on scRNA-seq data, appropriate flow cytometry sorting strategies are devised. Flow cytometry was used to sort pathogenic cell subpopulations, which are subsequently co-cultured in vitro with fibroblast-like synoviocytes (FLS). The expression of IL-6, MMP1/2/13 in FLS is determined using qPCR.
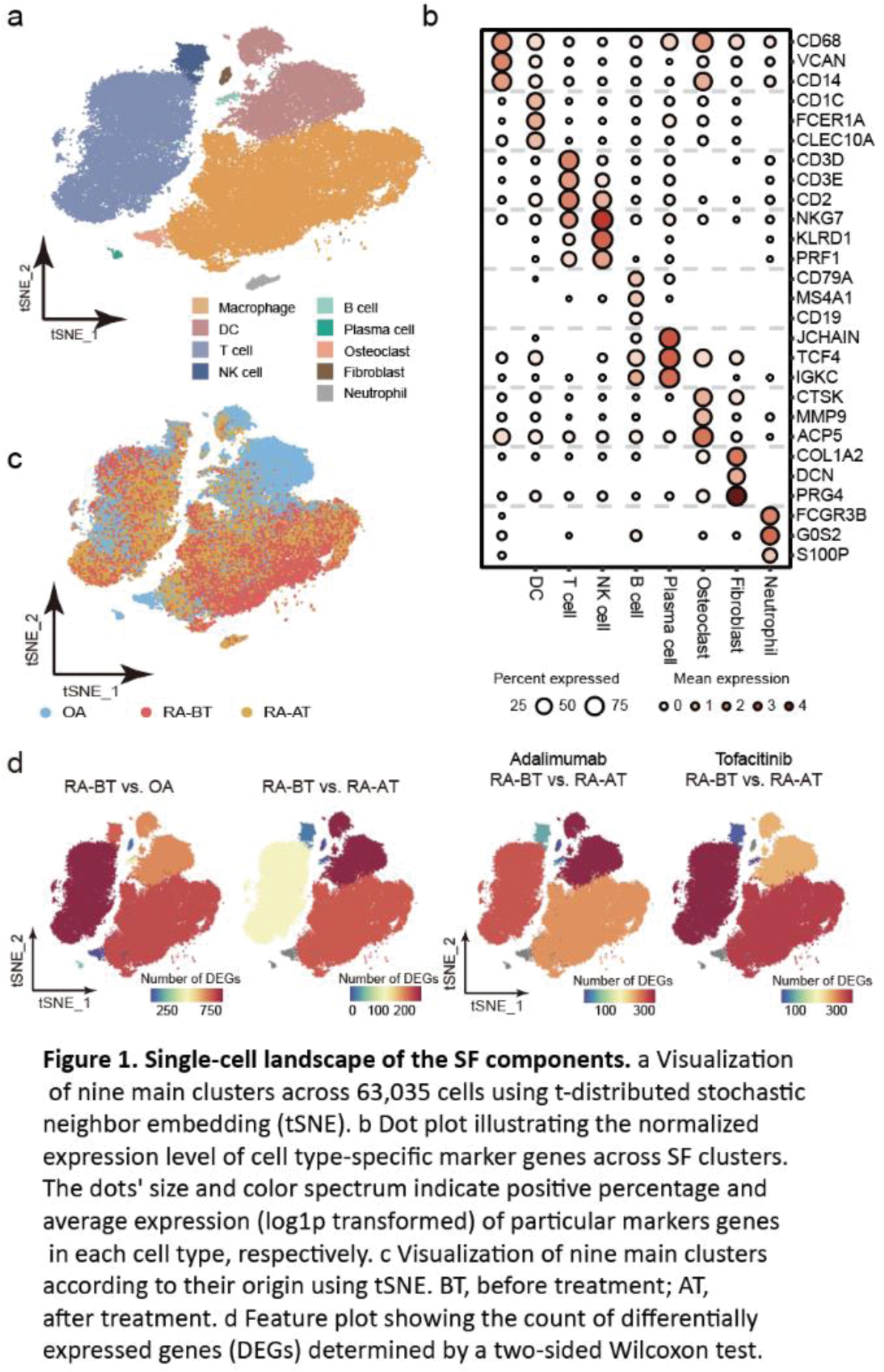
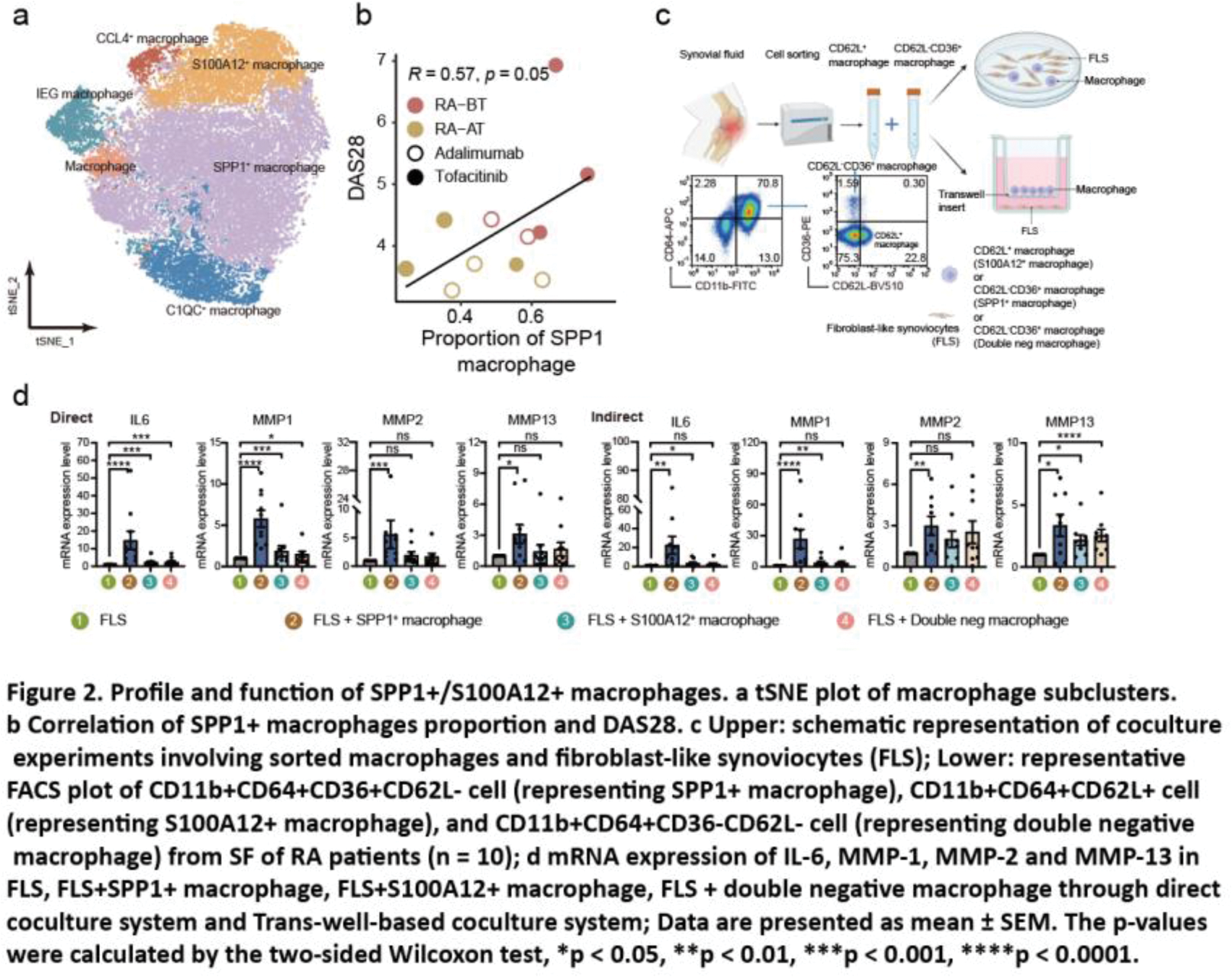
Results: After quality control and batch effect correction of scRNA-seq data, 63,035 cells from SF were clustered into nine main cell types with an unsupervised approach (Figure 1a), defined by well-established canonical marker genes (Figure 1b). In terms of cellular components, SF from RA patients was dominated by macrophages and T cells, especially macrophages. SF from OA patients had the highest proportion of dendritic cells (DCs) (Figure 1c). Moreover, we found that there are a large number of differential expression genes (DEG) in macrophages and T cells, not only between the RA-BT and the OA group, but also between the RA-BT and RA-AT group. It suggested that macrophages and T cells are necessary to be considered as candidate cell populations to further molecular characterization and functional profiling (Figure 1d). To characterize the role of macrophages in RA pathogenesis and treatment, we defined six transcriptionally distinct macrophage subtypes (Figure 2a), including IFN-activated SPP1 + macrophages (expressing high levels of SPP1 , CCL2 , and STAT1 ), and S100A12 + macrophages (expressing high levels of inflammation-triggering alarmins S100A8/9/12 ). These two macrophage subpopulations have higher cell densities in SF from RA patients than OA and decreased significantly after treatment. For SPP1 + macrophages, the proportion was also positively correlated with DAS28 (Figure 2b). In addition, we developed a flow cytometry sorting strategy for SPP1 + /S100A12 + macrophages based on scRNA-seq data. CD11b + CD64 + CD36 + CD62L - was used to represent SPP1 + macrophages and CD11b + CD64 + CD62L + to represent S100A12 + macrophages. To establish the pathogenic role of these two types of SF-derived macrophages, we cocultured them with FLS (Figure 2c). After coculturing in a direct and indirect system, the expression of IL6 and MMPs in FLS were significantly stimulated by both cell types compared to FLS alone or coculture with double negative macrophages (i.e., CD36 - CD62L - cells) (Figure 2d), suggesting FLS can be activated by SPP1 + / S100A12 + macrophages without the need for cell contact.
Conclusion: Our study mapped the single cell immunoprofile of SF in RA patients with TNF-α/JAK inhibitor treatment. We also identified two potentially pathogenic macrophage subpopulations in the SF from RA patients, which were confirmed to induce FLS activation in vitro. These may provided a reference for identifying new therapeutic targets for RA, investigating the mechanisms of action of TNF-α/JAK inhibitors.
REFERENCES: [1] Zhang F , et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nature immunology 20 , 928-942 (2019).
[2] Alivernini S , et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nature medicine 26 , 1295-1306 (2020).
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (