

Background: Although not part of the formal ACR criteria for rheumatoid arthritis (RA), psoriatic arthritis (PsA), or osteoarthritis (OA), synovial pathology can be a helpful tool in clinical practice. Histopathological evaluation of patients with RA has led to the creation of a synovitis score and a joint pathology algorithm which can be applied to characterize synovitis [1]. Three synovial pathotypes have been described in RA: lympho-myeloid, diffuse-myeloid, and pauci-immune [2]. Even though these pathotypes have been consistent between studies and can be useful, they are based on a limited number of markers and currently provide limited clinical information. Technological advancements have allowed for minimally invasive synovial biopsies in patients with arthritis. Innovation in microscopy allows multiple targets to be studied simultaneously in the tissue, providing more mechanistic pathology insights. This is particularly important, as satisfactory targeted therapies for osteoarthritis are pending. Artificial intelligence (AI) allows for a thorough image analysis of the tissue, preserving the spatial context, providing digital quantification of single cell data, and identifying cellular patterns and interactions within the synovial tissue that may ultimately allow for targeted therapy strategies and prediction of treatment response.
Objectives: To apply a novel high plex platform coupled with a semi-automatized immune analysis approach based on machine learning algorithms to quantify synovial inflammatory infiltrates in synovial biopsies of patients with PsA, RA, and OA.
Methods: Formalin fixed paraffin embedded (FFPE) synovial tissue biopsies from nine patients with RA, OA, and PsA were stained with an IF 17-marker panel with antibodies for nuclei, CD31, CD68, CD163, CD20, CD4, CD8a, CD45RO, PD-L1, CD3e, Ki-67, PD-1, FOXP3, CD45, Pan-CK, vimentin, SMA. Samples of RA and PsA patients were previously stained using conventional IF and IHC for CD68, CD3, CD20, CD138 and classified as having either a lymphoid (n=4) or lympho-myeloid (n=3) type of infiltrate [3]. Whole tissue sections were scanned in high resolution at 20x with Orion slide scanner (Figure 1). We applied a semiautomated image analysis strategy based on AI initially developed to study pancreatic human tissue [4]. In synovial biopsies, regions of interest were selected and classified as lining or sublining. Cell detection and cell classification based on characteristics of the fluorescent signal was performed with a semi-automated approach using supervised machine learning algorithms. Spatial context was considered in the analysis. Additionally, unsupervised machine learning algorithms are currently being processed to analyze spatial distribution of cellular clusters and cellular neighborhoods in the regions of interest described above.
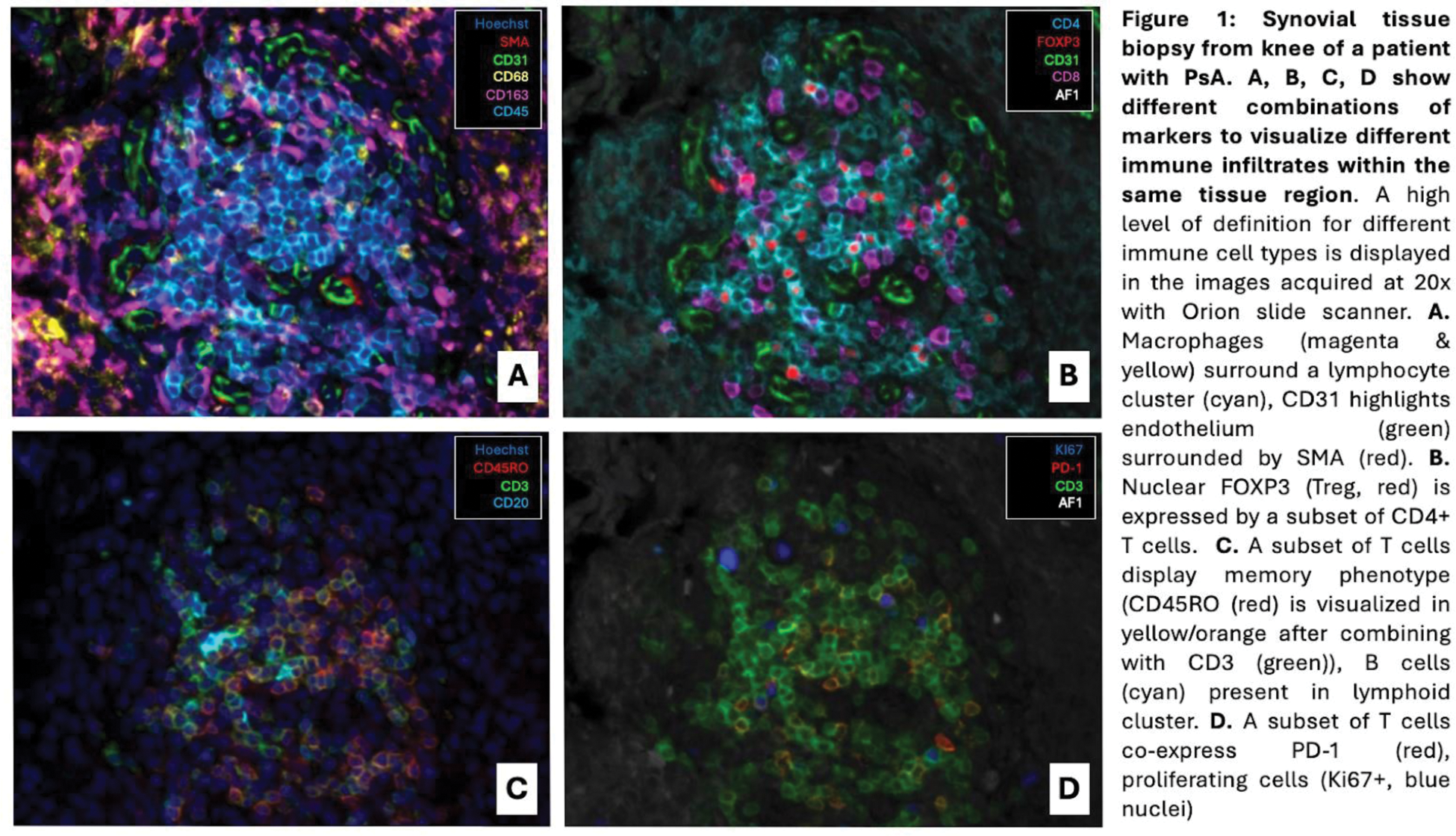
Results: Nine synovial biopsies were analyzed of patients with RA (n= 5, 3 knees and 2 wrists), OA (n=2, knees), and PsA (n=2, knees). Tissue sections were obtained from five male patients and five female patients with a mean age of 59.1 years (SD 10.1). A total of 114,900 cells were analyzed in 160 regions of interest within the synovium (76 sublining and 84 lining regions). The total tissue area analyzed was 23.7 mm 2 . The cellular density was similar for all patients regardless of disease, and our data overall showed a higher cell density in the lining regions (mean 10,461 cells/mm 2 with SD 2,124) compared to sublining regions (mean 5,017 cells/mm 2 with SD 3,436). As expected, immunofluorescence revealed less immune infiltrates (i.e. CD3+ T cells, CD20+ B cells, CD68+ macrophages), fibroblasts, and vascularization in OA compared to RA and PsA. In all patients, regardless of the presence of T and B cells, there was an overall predominance in macrophage infiltration and fibroblasts, which was more relevant in the immunomediated inflammatory arthritis. In RA patients, T cell infiltration was more abundant in the sublining compared to the lining and proportion of immune infiltrates for the various immune cell subtypes (i.e. CD3+, CD4+, FOXP3+ Tregs and CD3+, CD4+, PD1+ exhausted T cells) in both wrists and knee were similar.
Conclusion: Our preliminary data showcase the suitability of this new microscopy technology coupled with our image analysis strategy in defining the inflammatory infiltrate in synovial biopsies of patients with RA, PsA, and OA. Even with very little tissue, we were able to get high quality data of thousands of cells in a thorough quantitative analysis that allowed for both supervised and unsupervised strategies based on AI. We are pending full quality control and spatial biology studies of the collected data. We envision that the type of data generated may be helpful in creating algorithms in joint pathology to further aid in patient management. Novel platforms currently restricted to research can be incorporated into the clinical arena to serve as a diagnostic tool as well as a personalized treatment tool based on a patient’s specific histological characteristics.
REFERENCES: [1] Krenn V et al. Pathol Res Pract . 2017;213(8):874-881. DOI: 10.1016/j.prp.2017.05.005
[2] Lliso-Ribera G et al. Ann Rheum Dis . 2019;78(12):1642-1652. DOI: 10.1136/annrheumdis-2019-215751.
[3] Murillo-Saich JD et al. Arthritis Rheumatol . 2024;76(8):1230-1242. doi:10.1002/art.42848
[4] Quesada-Masachs E et al. Diabetologia . 2022;65(2):387-401. doi:10.1007/s00125-021-05619-9
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (