

Background: Pregnancy in women with systemic lupus erythematosus (SLE) is associated with a high risk of adverse pregnancy outcomes (APOs), such as hypertensive disorders of pregnancy (HDP), which may have devastating effects on the mother and fetus. Changes in immune cells associated with APOs in patient with SLE are incompletely understood.
Objectives: The aim of this study was to identify characteristics of patients with SLE and APOs from mid-pregnancy using single-cell transcriptome RNA sequencing (scRNA-seq) of peripheral blood.
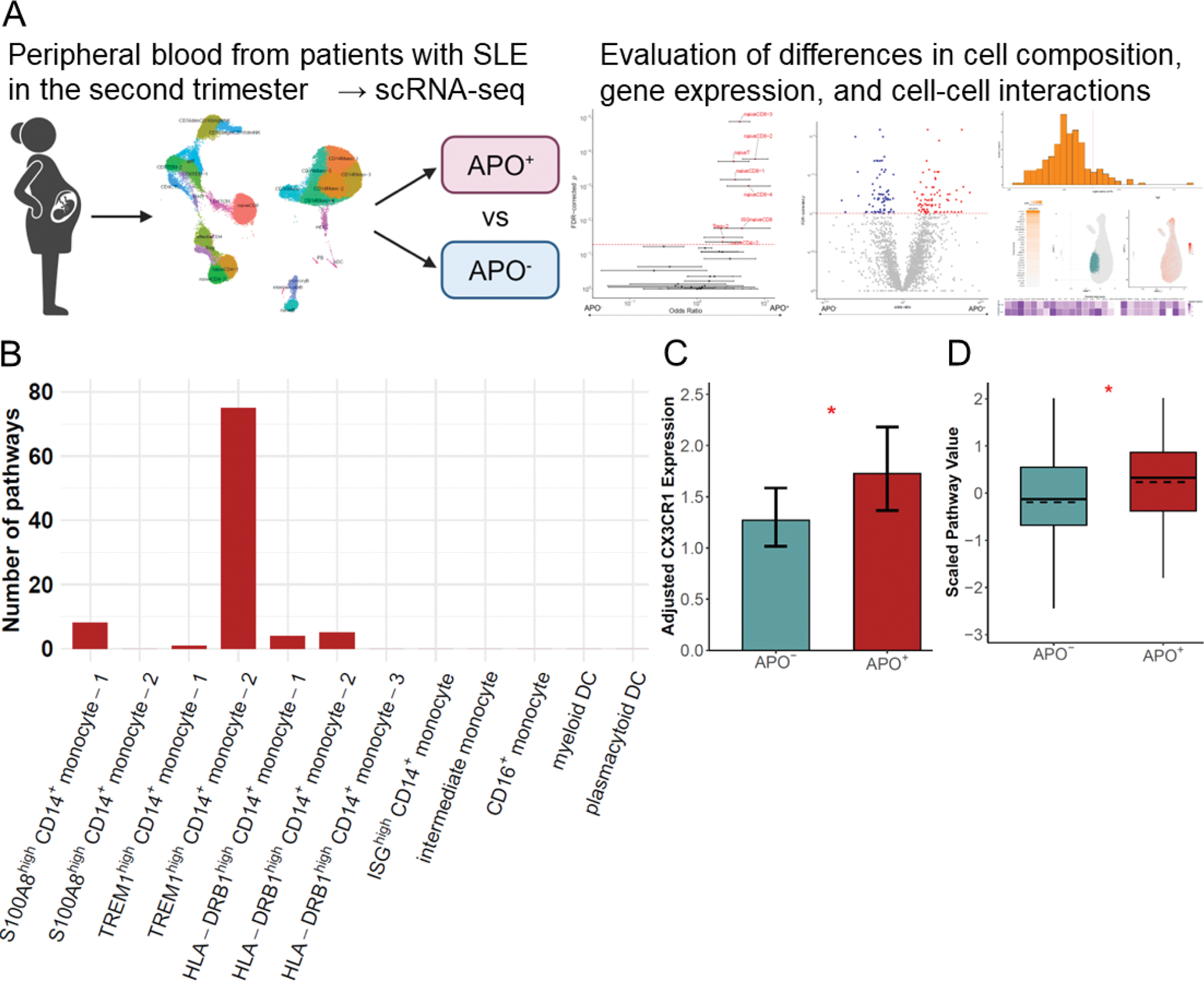
Methods: Peripheral blood mononuclear cells (PBMCs) were collected during the second trimester from 31 pregnant women with SLE and analyzed using scRNA-seq. Clinical data were obtained through the postpartum and cases were classified based on the presence or absence of APOs. APOs were defined as any of the following: HDP, birth weight < −1.5 standard deviations or 2,500 grams; or delivery at < 37 weeks gestation (Figure 1A). Mixed-effects association of single cells (MASC) [1], differentially expressed gene (DEG) analysis, and single-cell gene set enrichment analysis (scGSEA) were used to determine the characteristics of PBMCs in the APO group with covariate adjustment via generalized linear mixed models (GLMMs). Cell-cell interactions were assessed using residuals adjusted for covariates and analyzed with NicheNet [2].
Results: Of the 31 pregnancies, 13 showed APOs (APO + group). DEG analysis comparing the APO + and APO - groups identified numerous DEGs in myeloid cells, while the number of DEGs in lymphoid cells was limited. DEG pathway analysis of triggering receptor expressed on myeloid cells (TREM)1 high CD14 + monocyte-2 showed an enrichment of various pathways, such as the regulation and production of tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), suggesting that this subpopulation obtains a pro-inflammatory phenotype in the APO + group (Figure 1B). In this subpopulation, scGSEA demonstrated heightened type I interferon (IFN)-mediated signaling pathway ( p = 0.04) in the APO + group. Next, MASC analysis identified a higher proportion of naive CD8 + T cells, including IFN-stimulated gene (ISG) high naive CD8 + T cells associated with T cell differentiation and activation, in the APO + group (FDR-corrected p = 0.02). Partially differentiated CD8 + T cells expressed chemokine receptors that interact with chemokines expressed in the placenta of patients with APO ( p < 0.05, Figure 1C), suggesting subsequent migration of these cells to the placenta. Enhanced cell-cell interactions mediated by TNF-α and IL-1 were observed between TREM1 high CD14 + monocyte-2 and naive CD8 + T cells in the APO + group. Changes were also observed in B cells of the APO + group, such as the upregulation of the plasmablast differentiation pathway in naive B and atypical B cells ( p = 0.01, Figure 1D); and a higher proportion of atypical B cells within the B cell compartment (FDR-corrected p = 0.10). Enhanced cell-cell interactions mediated by type II IFN were observed between partially differentiated CD8 + T cells and naïve B cells in the APO + group.
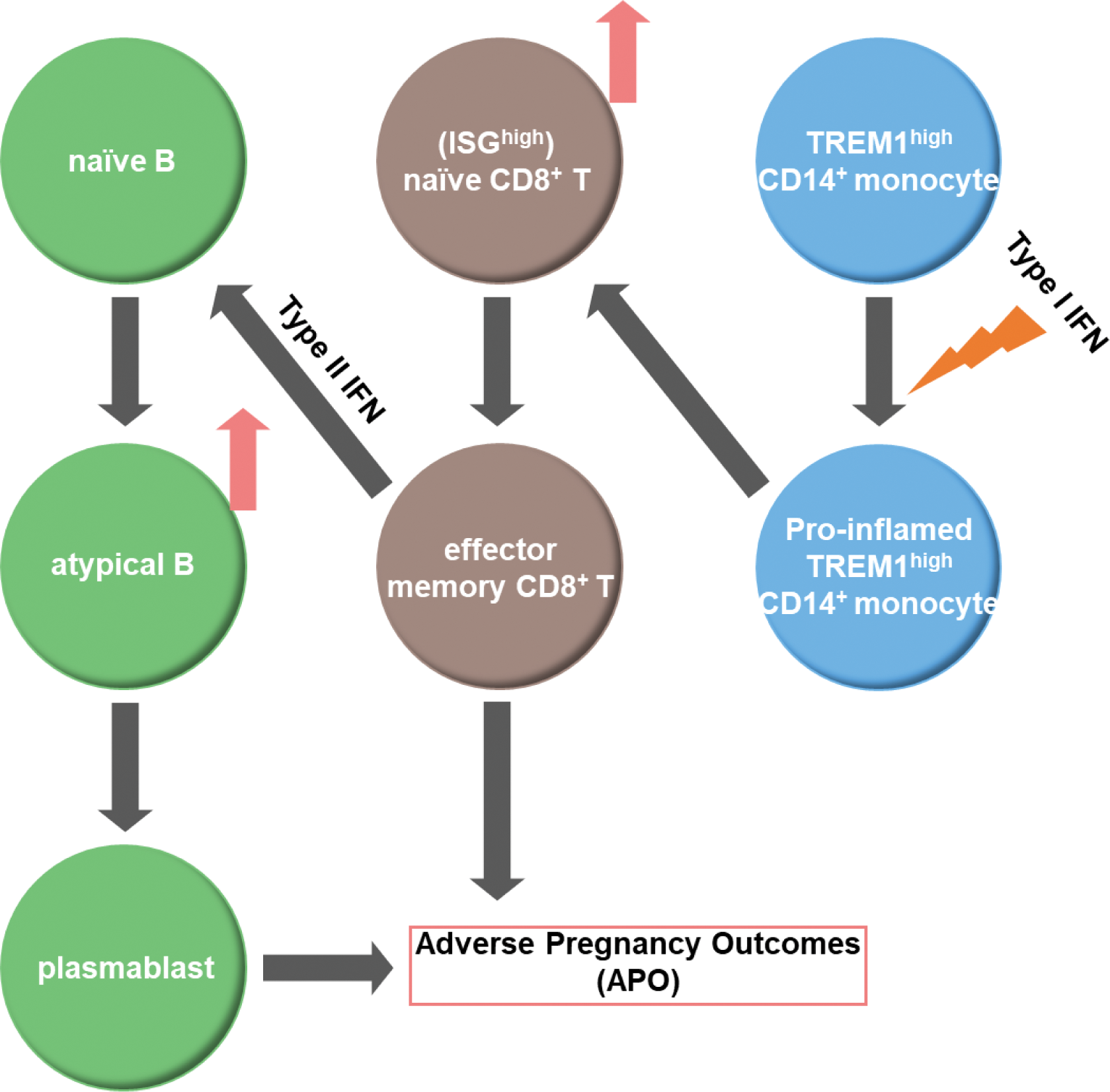
Conclusion: The current study demonstrated qualitative and quantitative changes in immune cells within the APO + group of PBMCs during the second trimester in pregnant women with SLE. TREM1 high CD14 + monocyte-2 appeared to have a pro-inflammatory phenotype driven by type I IFN in the APO + group, which may contribute to an increase in naive CD8 + T cells and their differentiation into effector memory CD8 + T cells. These CD8 + T cells interact with B cells, which may lead to an increase in atypical B cells and an upregulation of plasmablast differentiation in the APO + group (Figure 2). Our findings may provide a foundation for identifying high-risk cases for APO, enabling intensive monitoring.
(A) Overview of the Study. Single-cell transcriptome RNA sequencing (scRNA-seq) of peripheral blood from patients with systemic lupus erythematosus (SLE) during the second trimester was performed to assess changes in immune cells associated with adverse pregnancy outcomes (APOs). (B) Differential expression analysis comparing the APO + and APO - groups was performed, and the number of gene ontology biological process pathways that were enriched in differentially expressed genes (FDR-corrected p = 0.1) and with at least 5 overlapping genes were compared among each subpopulation. (C) A zero-inflated negative binomial generalized linear model was used to test for the association of CX3C motif chemokine receptor 1 (CX3CR1) expression levels in granzyme K + granzyme B - effector memory CD8 + T cells with APO + group (likelihood ratio test, * p < 0.05). Estimated marginal means and standard errors are presented. (D) Adjusted single-cell gene set enrichment analysis scores for plasma cell differentiation (GO: 0002317) in atypical B cells, stratified by APO status (Solid line: Median and IQR. Dashed line: mean. Wilcoxon test. * p < 0.05).
Immunological Changes Leading to Adverse Pregnancy Outcomes. Triggering receptor expressed on myeloid cells (TREM)1 high CD14 + monocyte-2 shifted to a pro-inflammatory phenotype in the APO + group, promoting the differentiation of IFN-stimulated genes (ISG) high naive CD8 + T cells into partially differentiated CD8 + T cells. These differentiated CD8 + T cells produce type II IFN and interact with naive B cells, inducing an increase in atypical B cells and plasmablast differentiation.
REFERENCES: [1] Fonseka CY et al. Mixed-effects association of single cells identifies an expanded effector CD4 + T cell subset in rheumatoid arthritis. Sci Transl Med 2018;10(463):eaaq0305
[2] Browaeys R et al. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods 2020;17(2):159-162
Acknowledgements: NIL.
Disclosure of Interests: Keigo Terada: None declared, Yumi Tsuchida AbbVie, Janssen, GSK, Astellas, Daiichi Sankyo, Eli Lilly, Novartis, Eisai, Asahi Kasei, AstraZeneca, Sotaro Nakajima: None declared, Sakiko Isojima: None declared, Takeo Isozaki Pfizer, Eli Lilly, AbbVie, Astellas, Eisai, Gilead, Chugai, UCB, Sanofi, Asahi Kasei, Taisho, Pfizer, Eli Lilly, AbbVie, Astellas, Esai, Gilead, Chugai, UCB, Sanofi, Asahi Kasei, Novartis, Taisho, Boehringger Ingelheim, Eli Lilly, Taisho, Eli Lilly, AbbVie, Otsuka, Asahi Kasei, Yuri Hiramatsu: None declared, Hiroshi Nakajima: None declared, Shinji Morimoto Chugai, Asahi Kasei Pharma, Ayumi, GSK, AstraZeneca, Chugai, Asahi Kasei Pharma, Nippon Kayaku, Toshihide Mimura: None declared, Naoto Tamura Asahi Kasei, AstraZeneca, AbbVie, Eli Lilly, GSK, Chugai, Novartis, Bristol Myers Squibb, Janssen, Asahi Kasei Pharma, Asahi Kasei Medical, Ayumi, AbbVie, Eisai, Nippon Boehringer Ingelheim, Taisho, Mitsubishi Tanabe, Chugai, Koichi Amano AbbVie, Astellas, AstraZeneca, Chugai, Eisai, Eli Lilly, GSK, Pfizer, Taisho, Asahi Kasei Pharma, Chugai, Yohei Kirino Amgen, Novartis, Sobi, Novartis, Amgen, Shigeru Ohno: None declared, Isao Matsumoto Asahi Kasei Pharma, Astellas, Nippon Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eisai, Eli Lilly, Pfizer, Shinsuke Yasuda Abbvie, Asahi Kasei Pharma, Chugai, Eisai, Eli Lilly, GSK, Mitsubishi Tanabe, Ono, Eisai, ImmunoForge, Novartis, Otsuka, Asahi Kasei Pharma, Ayumi, Chugai, Nippon Boehringer Ingelheim, Taisho, Yuko Kaneko: None declared, Masayoshi HARIGAI: None declared, Shinya Kaname Teijin Pharma, Teijin Health Care, Kissei, Chugai, AstraZeneca, Mitsubishi Tanabe, Kyowa Kirin, Novartis, Daiichi Sankyo, Alexion, Kissei, Alexion, Daiichi Sankyo, Nippon Kayaku, Eisai, Kissei, Teijin Pharma, Chugai, Mitsubishi Tanabe, AbbVie, Astellas, Mochida, Kyowa Kirin, Nippon Boehringer Ingelheim, Otsuka, Torii, Asahi Kasei Pharma, Sanofi, Kenji Itoh Eli Lilly, Chugai, Asahi Kasei Pharma, Taisho, Hideto Kameda AbbVie, Asahi Kasei, Bristol Myers Squibb, Eisai, Eli Lilly, Janssen, Mitsubishi Tanabe, UCB, AbbVie, Amgen, Asahi Kasei, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Taisho, UCB, Pfizer, Kayoko Kaneko Chugai, Astellas, Tomohisa Okamura Asahi Kasei, Bristol Myers Squibb, Chugai, Yukinori Okada: None declared, Atsuko Murashima Astellas, UCB, Chugai, Asahi Kasei Pharma, Keishi Fujio Asahi Kasei Pharma, Chugai, Abbvie, Asahi Kasei Pharma, Bristol Myers Squibb, AstraZeneca, Mitsubishi Tanabe, Eisai, Gilead, Eli Lilly, Pfizer, Taisho, Astellas, Daiichi Sankyo, Novartis, GSK, Alexion, Asahi Kasei Pharma, Chugai, Abbvie, Asahi Kasei Pharma, Bristol Myers Squibb, AstraZeneca, Eisai, Tsumura, Taisho, Nobuyuki Yajima: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (