

Background: Juvenile localized scleroderma (JLS) is a rare autoimmune connective tissue disorder characterized by inflammation and fibrosis of the skin affecting children under the age of 16 years. Histopathologic studies of affected skin have identified as hallmarks of JLS sclerosis (i.e. deposition of collagen) of the dermis and dermal infiltration of inflammatory cells, suggesting a link between inflammation and activation of fibroblasts to produce excess extracellular matrix (Walker D, J Am Acad Dermatol. 2017). Clinical examination and/or musculoskeletal imaging are not always able to distinguish active from inactive lesions and there is a need for biomarkers able to better assess the degree of activity and predict response to therapy. Determining immune mediators within peripheral blood, with a key role in the pathogenesis of JLS, would improve disease activity detection and clinical management of JLS.
Objectives: The aim of this project is to perform immunophenotyping of patients with JLS and healthy controls to assess dysregulated immunological pathways in JLS, and to identify biomarkers able to predict clinical features.
Methods: We included in this study pediatric patients diagnosed with linear and mixed JLS. Peripheral blood mononuclear cells (PBMCs) and plasma samples at diagnosis or treatment escalation were identified retrospectively and retrieved from our biobank. We included only patients whose first available blood sample was obtained at time of diagnosis and before the initiation of any treatment. Levels of 50 cytokines were measured in the plasma by protein array (RayBiotech). Lymphocyte and monocyte subsets were analysed with a 26-colour flow cytometry panel and acquired on a FACSymphony (BD).
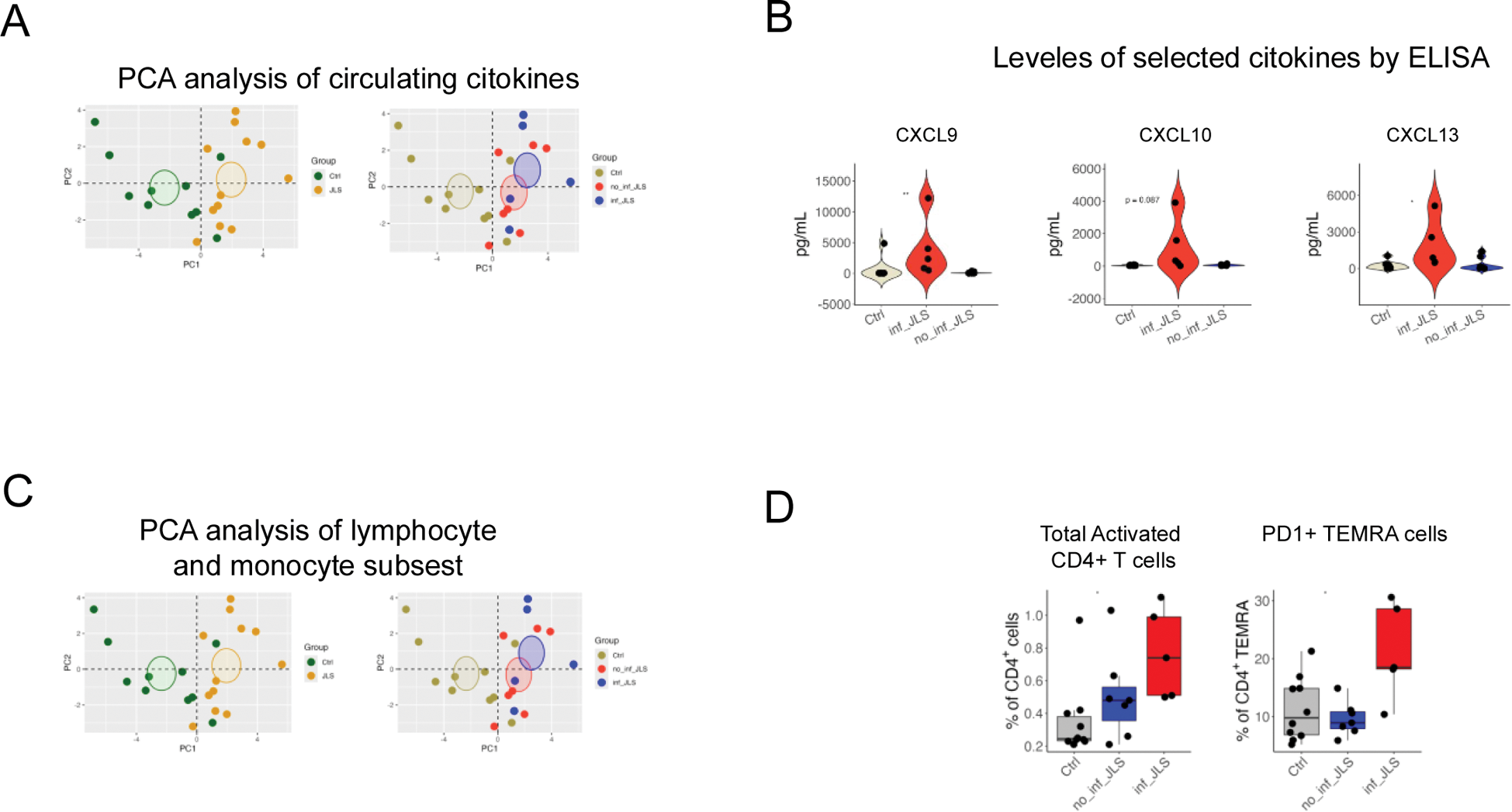
Results: We identified samples at baseline from 11 patients with JLS and 10 controls. Cytokine array identified 15 cytokines that were significantly elevated in JLS patients compared to controls. Using Principal Component Analysis (PCA), we observed a distinct distribution between patients and controls, with approximately 50% of patients clustering away from controls, and the remaining 50% aligning closely with them (Figure 1A). Patients clustering near controls exhibited the lowest cytokine levels and were designated as non-inflammatory JLS (no_inf JLS), whereas those clustering away had the highest cytokine levels and were classified as inflammatory JLS (inf_JLS) (Figure 1A). We then validated the results of the cytokine array in ELISA, focusing on the most elevated cytokines observed in patients with inf_JLS: CXCL9, CXCL10, CXCL13, TNF, IL-6, and IL-8. Of these, CXCL9, CXCL10, and CXCL13 were consistently elevated in all patients with inf_JLS, at baseline and during longitudinal follow-up, while cytokine levels in patients with no_inf JLS remained within normal ranges (Figure 1B). We then performed a 26-parameter staining of PBMCs, allowing comprehensive evaluation of major lymphocytes and monocytes subsets by flow cytometry. Our analysis identified 81 subpopulations of interest; a PCA analysis highlighted significant differences in the distribution of lympho-monocytic subsets between JLS patients and controls (Figure 1C). We observed a reduction in monocyte populations, including classical (CD14 + CD16 − ) and atypical monocytes (CD14 + CD16 + ) and an expansion of dendritic cells compared to controls. Within lymphocyte subsets, significant differences were observed in the frequency of CD4 + T cells, particularly of activated CD4 + T cells (CD38 + HLA-DR + ), predominantly within the central memory (CM) subset. Patients stratified into inf_JLS and no_inf JLS groups based on cytokine levels showed minimal differences in cellular subpopulations upon PCA. However, two subpopulations demonstrated significant variability: activated CD4 + T cells (CD38 + HLA-DR + ) and terminally differentiated effector memory CD4 + T cells (TEMRA) expressing PD1, were significantly expanded in patients with inf_JLS (Figure 1D).
Conclusion: This study identified two distinct groups of patients with JLS: a non-inflammatory group (no_inf JLS), characterized by normal levels of circulating proinflammatory cytokines, and an inflammatory group (inf_JLS), marked by elevated levels of circulating cytokines. While all JLS patients exhibited alterations in monocyte and T cell subsets, additional abnormalities were observed specifically in the inf_JLS group. These included higher levels of activated CD4 + T cells, changes in central memory (CM) T cells, and alterations in terminally differentiated CD4 + T cells (TEMRA PD1 + ), a phenotype associated with cellular exhaustion. These findings highlight the central role of activated T cells in JLS pathogenesis, particularly in patients within the inflammatory group, and the possible role of circulating cytokines and T cell subpopulation as biomarker of disease. Furthermore, these results pave the way for novel therapeutic approaches for JLS, including the potential use of treatments targeting T cells.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Emiliano Marasco BMS, Angela Aquilani: None declared, Ivan Caiello: None declared, Fabrizio De Benedetti SOBI, Novartis, Rebecca Nicolai: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (