

Background: Antiphospholipid syndrome (APS) is a systemic autoimmune condition characterised by the presence of antiphospholipid antibodies (aPL) associated with vascular thrombosis (TAPS) or pregnancy complications (OAPS). We and others have described a different profile of aPL (including criteria and non-criteria) positivity between both clinical phenotypes [1, 2]. Moreover, monocytes cultured with aPL from OAPS or TAPS patients showed a different gene and protein expression [3, 4]. All these data eventually suggest pathogenic mechanisms specific to OAPS and different from TAPS.
Objectives: We aimed to observe whether the aPL-dependent activation is distinct in immune cells from OAPS and TAPS patients.
Methods: In this study we investigated how aPL trigger distinct cellular signalling pathways by examining epigenetic and inflammatory markers in flow cytometry-sorted monocytes from 9 OAPS, and 9 TAPS patients, and compared them to monocytes from 8 healthy women (HD). Immune profile of circulating cells from both groups was done by multicolour flow cytometry.
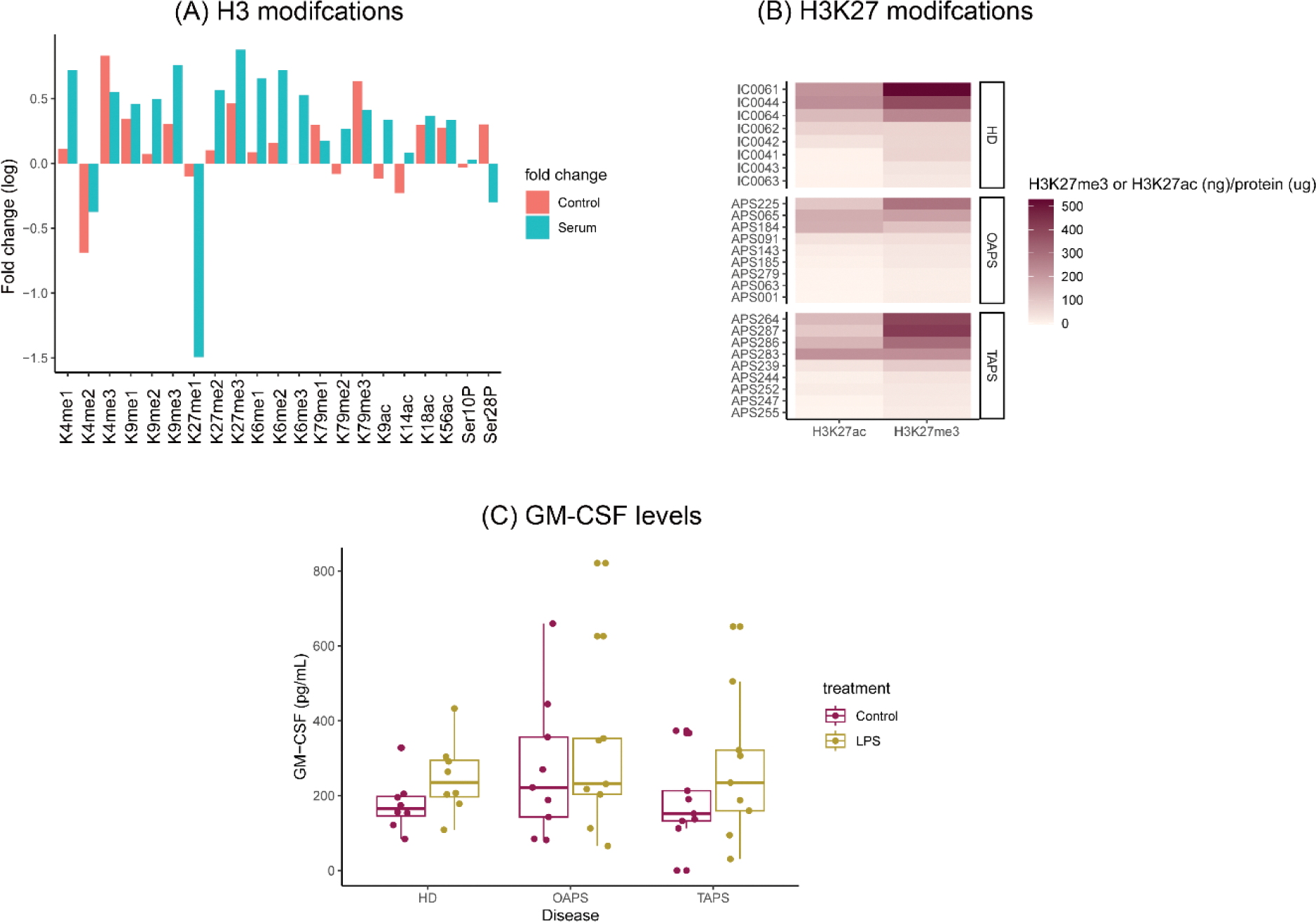
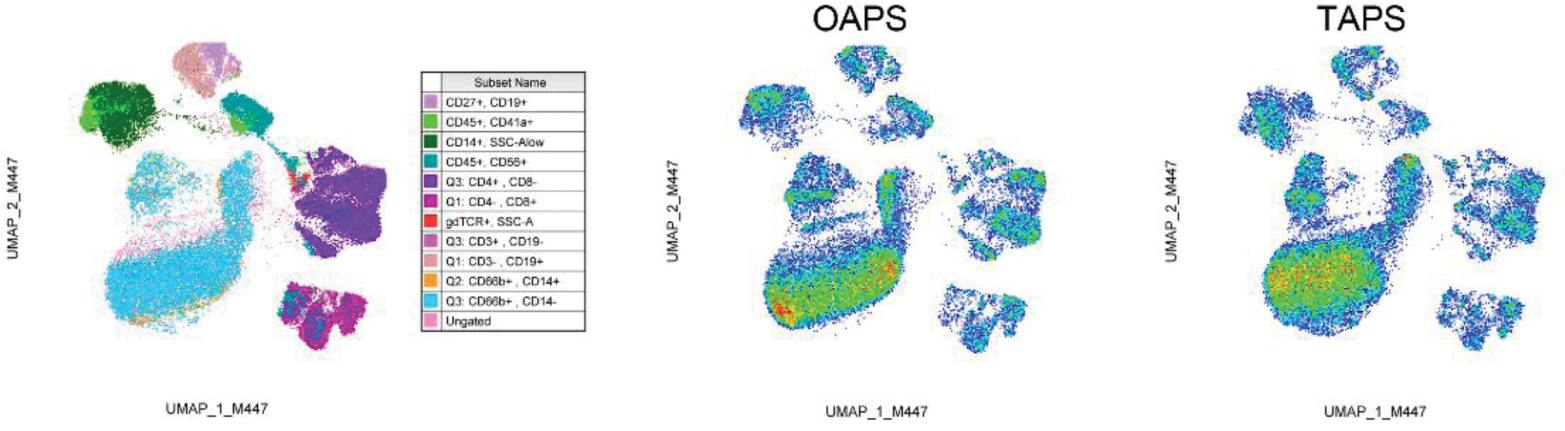
Results: First, 21 epigenetic markers were screened in monocytes treated or not with serum from APS patients and we found that APS sera induced differential epigenetic changes (Figure 1A). We then explored differential epigenetic modifications among OAPS, TAPS, and HD monocytes at the basal level. Although monocytes showed a highly diversified profile of these epigenetic markers, we observed less H3K27me3 and H3K27ac in monocytes from OAPS patients (Figure 1B). These findings associated with higher basal levels of GMCSF in this same group of monocytes that did not overproduced GMCSF when stimulated with LPS or APS serum (Figure 1C). We are wondering whether these epigenetic modifications could alter, for instance, GMCSF release by monocytes that in turn affect neutrophil recruitment to the endometrium in pregnancy. Finally, we immunophenotyped circulating cells from the three group of individuals, and found a differential phenotype in monocytes from OAPS patients (Figure 2).
Conclusion: OAPS and TAPS presented differences in epigenetic markers indicating different chromatin regulation. This affected inflammatory and myelocytic gene transcription. These data could provide insights into the molecular and cellular mechanisms underlying OAPS, and potential specific biomarkers or therapeutic targets.
REFERENCES: [1] Anunciación-Llunell A, et al. Differences in Antiphospholipid Antibody Profile between Patients with Obstetric and Thrombotic Antiphospholipid Syndrome. Int J Mol Sci 2022;23:12819.
[2] Park HS, Gu JY, Jung HS, Kim HK. Thrombotic risk of non-criteria anti-phospholipid antibodies measured by line immunoassay: Superiority of anti-phosphatidylserine and anti-phosphatidic acid antibodies. Clin Lab 2019;65:207–13.
[3] Ripoll VM, et al. Changes in regulation of human monocyte proteins in response to IgG from patients with antiphospholipid syndrome. Blood 2014;124:3808–16.
[4] Ripoll VM, et al. Gene expression profiling identifies distinct molecular signatures in thrombotic and obstetric antiphospholipid syndrome. J Autoimmun 2018;93:114–23.
Differences in epigenetic markers and inflammatory cytokines in OAPS and TAPS patients. (A) aPL-bearing serum did induce epigenetic modifications in monocytes that are associated with gene expression or gene repression. (B) Epigenetic markers related to open (H3K27ac) and close (H3K27me3) chromatin structure that allows transcriptional machinery to access DNA, were differentially detected in monocytes from OAPS and TAPS patients. (C) At the steady state, monocytes from OAPS patients produced higher levels of GM-CSF than monocytes from TAPS patients. Two-way ANOVA with Tukey post-hoc analysis.
Immune cells population in OAPS and TAPS patients. Flow cytometry-based, uniform manifold approximation and projection (UMAP) analysis of circulating immune cells from OAPS and TAPS patients. Immune cells are identified in the left UMAP plot. Monocytes are depicted by the expression of CD45 and CD14 and pictured in dark green.
Acknowledgements: Gravida Fertilitat Avançada (Barcelona, Spain, Gravida-JAR/2023); UAB-Gravida Catedra Medicina i immunologia reproductiva (Barcelona, Spain, CatGravida-JAR/2024); Synlab (Barcelona, Spain, Synlab-JAR/2023).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (