

Background: ANCA-associated vasculitis (AAV) is characterized by a loss of B cell tolerance, leading to the production of ANCAs that activate neutrophils and monocytes and cause organ damage [1]. While anti-CD20 therapy with rituximab is effective, up to 30% of patients fail treatment, and over 40% relapse, requiring lifelong therapy [2, 3]. Some relapse even within 18 months of fixed schedules [4]. Alternative treatments like anti-CD19 CAR T cells may benefit refractory cases.
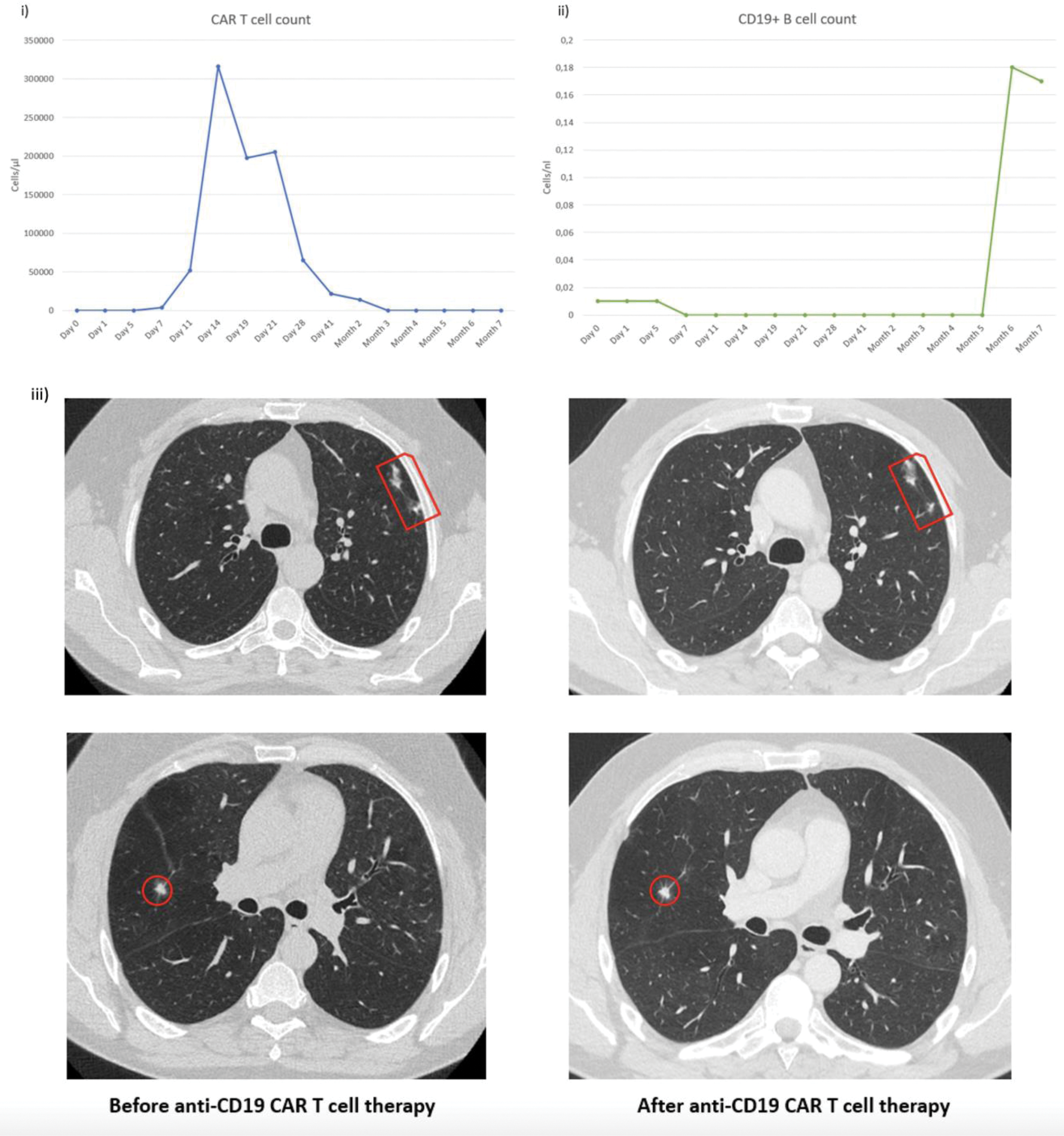
Case presentation: A 52-year-old male with 20 years of severe, treatment-refractory (cyclophosphamide, rituximab, mycophenolate mofetil, azathioprine and methotrexate) PR3-ANCA+ AAV involving multiple organs (lungs, kidneys, joints, skin, sinonasal tract, eyes) presented with fever, weight loss, myalgia, arthralgia, exertional dyspnea, and productive cough (BVAS 6) despite repetitive treatment with rituximab over the past 10 years. He showed minimal CD19+ B cells and elevated PR3-ANCA (121.1 AU/ml). Reinduction therapy with rituximab and avacopan partially improved dyspnea and stabilized weight, but myalgia and arthralgia persisted (BVAS 2). Due to relapsing disease, he received 1x10 8 anti-CD19 CAR T cells following lymphodepletion with cyclophosphamide and fludarabine. CAR T cells expanded rapidly, peaking on day 14 (44.46% of CD3 cells) and declining over six weeks. He developed grade 1 CRS managed with 3 doses of tocilizumab and grade 3 neutropenia, resolved with filgrastim. No ICANS, infections, or other toxicities occurred. Symptoms resolved, granulomas stabilized, and BVAS dropped to 0 at 132 days post-therapy. CD19+ B cells disappeared after 7 days and reemerged after 6 months. At 7 months, the patient remained symptom-free (BVAS 0) without immunosuppressive treatment despite the return of CD19+ B cells.
Learning points for clinical practice: Anti-CD19 CAR T cell therapy could be a safe and promising treatment option for the small subset of AAV patients who continue to experience recurrent relapses despite ongoing rituximab therapy.
REFERENCES: [1] Kitching AR et al. Nat Rev Dis Primers. 2020.
[2] Smith RM et al. Arthritis Rheum. 2012.
[3] Pagnoux C et al. Arthritis Rheum. 2008.
[4] Guillevin L et al. N Engl J Med. 2014.
Circulating anti-CD19 CAR T (i) and B cell count (ii) from day 0 to month 7, iii) Chest CT before rituximab reinduction and 4 months after anti-CD19 CAR T cell therapy showing constant granulomas indicated by red circles and squares.
Acknowledgements: NIL.
Disclosure of Interests: Ioanna Minopoulou AbbVie, Novartis, Lilly, Artur Wilhelm: None declared, Fredrik N. Albach: None declared, Arnd Kleyer: None declared, Edgar Wiebe: None declared, Simon Schallenberg: None declared, Anja Fleischmann: None declared, Mareike Frick: None declared, Frederik Damm: None declared, Julia Gogolok: None declared, Sebastian Serve: None declared, Benjamin Nick Locher: None declared, Dominic Borie Kyverna Therapeutics, Vincent Casteleyn: None declared, Robert Biesen: None declared, Thomas Dörner AbelZeta, BMS, J&J, Novartis, Roche/Genentech, Elpida Phitak: None declared, Norman Michael Drzeniek: None declared, Tobias Alexander: None declared, Jan Zernicke: None declared, Kamran Movassaghi: None declared, Marie Luise Hütter-Krönke: None declared, Eva Schrezenmeier: None declared, Adrian Schreiber: None declared, Udo Schneider: None declared, Lars Bullinger: None declared, Gerhard Krönke: None declared, Olaf Penack Alexion, Gilead, Jazz, MSD, Neovii, Novartis, Pfizer and Therakos, David Simon AbbVie, Alfasigma, Bristol-Myers Squibb, Janssen-Cilag, Lilly, Novartis, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (