

Background: Recent studies highlight the utility of sacrum MRI in diagnosing axial spondyloarthritis (axSpA). The Assessment of SpondyloArthritis International Society (ASAS) criteria define positive MRI findings based on the presence of bone marrow edema (BME) in the sacroiliac joints. However, BME may lead to false positives in situations such as post-exercise or postpartum and false negatives in chronic axSpA cases. Chronic axSpA often exhibits structural changes like erosion, ankylosis, and fat deposition, but criteria for categorizing these changes as indicative of axSpA remain unclear. While artificial intelligence (AI) has shown promising performance in medical imaging diagnostics, previous studies on axSpA have mainly focused on detecting specific imaging features, particularly BME, rather than predicting clinical diagnoses. Considering that clinicians prioritize clinical diagnosis over individual imaging findings, integrating structural features with BME may improve diagnostic accuracy for axSpA.
Objectives: This study aims to propose a deep learning model that integrates inflammatory and structural changes in sacrum MRI to address the gap between BME detection and clinical diagnosis of axSpA. By reducing reliance on BME and evaluating comprehensive pathological features, this approach seeks to enhance diagnostic accuracy and efficiency.
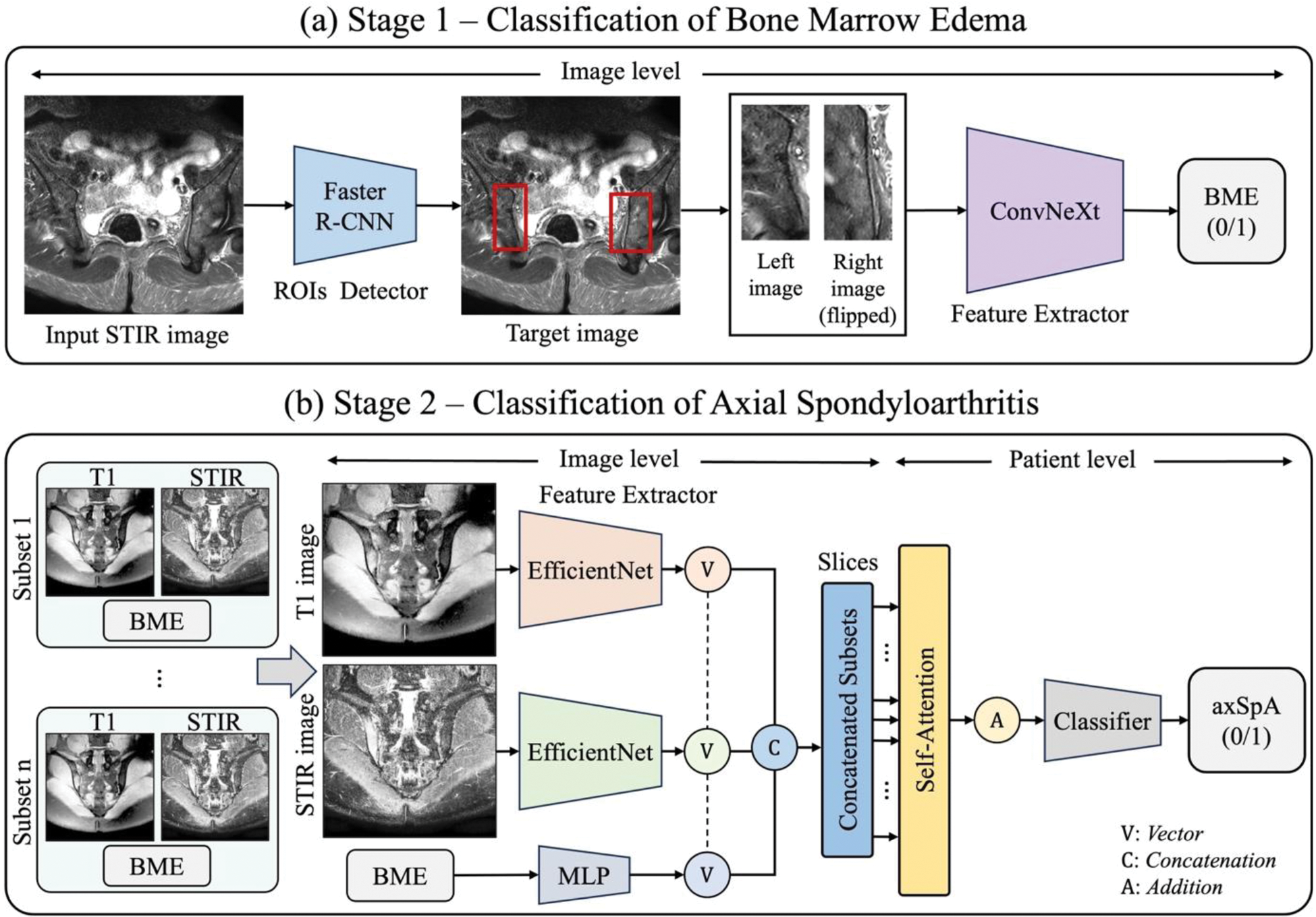
Methods: Data were collected from patients with chronic back pain who underwent sacrum MRI at Samsung Medical Center from January 2010 to December 2021. Clinical diagnosis of axSpA was defined based on ASAS criteria for axSpA. BME was identified using the ASAS definition: presence of ≥2 lesions in one image or 1 lesion in at least two images. An end-to-end deep learning framework (Figure 1) was developed, utilizing short tau inversion recovery (STIR) and T1-weighted MRI sequences to reflect inflammatory and structural changes, respectively. In Stage 1 (Figure 1a), Faster R-CNN detected regions of interest (ROIs) in the sacroiliac joints, and ConvNeXt identified BME within these ROIs. In Stage 2 (Figure 1b), features from STIR and T1-weighted MRI were extracted using an EfficientNet-based network to capture inflammatory and structural changes. Simultaneously, BME data were processed using a multi-layer perceptron (MLP). These features were integrated to classify axSpA, combining slice-level information for final diagnosis. Model performance was evaluated using accuracy, sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) metrics.
Results: MRI data from 291 patients, with an average of 14 slices per patient (total: 4,086 slices), were analyzed. Among the participants, 163 were clinically diagnosed with axSpA, while 128 had nonspecific back pain. The BME detection model achieved a sensitivity of 81.62%, specificity of 82.00%, AUROC of 0.82, and accuracy of 81.75% (Table 1a). The axSpA classification model demonstrated a sensitivity of 87.5%, specificity of 84.0%, AUROC of 0.94, and accuracy of 85.96% (Table 1b). Notably, the model identified 6 out of 9 axSpA patients who met clinical criteria but not ASAS-defined positive MRI criteria, indicating its ability to detect features beyond conventional criteria.
Conclusion: This study demonstrated that integrating STIR and T1-weighted MRI with BME data effectively reflects both inflammatory and structural changes, enabling the AI model to predict clinical diagnoses of axSpA. Additionally, the proposed model successfully identified axSpA patients who did not meet the ASAS criteria for positive MRI, proving its ability to comprehensively analyze pathological features of sacrum MRI beyond mere reliance on BME. The end-to-end deep learning framework proposed in this study reduces dependency on BME and emphasizes the value of multimodality analysis for a more comprehensive evaluation in axSpA diagnosis. These findings highlight the potential of AI not only to detect specific imaging features but also to predict clinical diagnoses, suggesting promising applications in real-world clinical practice.
REFERENCES: NIL.
Overview of the proposed model.
Result of the BME classification and axSpA classification
| (a) BME Classification | (b) AxSpA Classification | |
|---|---|---|
| Accuracy | 0.8175 (233/285) | 0.8596 (49/57) |
| Sensitivity | 0.8162 (151/185) | 0.8750 (28/32) |
| Specificity | 0.8200 (82/100) | 0.8400 (21/25) |
| AUROC | 0.8181 | 0.9412 |
Acknowledgements: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00439690).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (