

Background: CD19-CAR T-cell treatment is a novel option of deep B-cell depletion with promising results in a first case series of patients with diffuse cutaneous Systemic sclerosis (dcSSc) [1, 2]. Larger studies with higher patient numbers, longer follow-up times and control groups are necessary to further characterize the effects of CD19-CAR T-cell treatment in dcSSc. The revised European Scleroderma Trials and Research Group Activity Index ((EUSTAR)-AI) and the American College of Rheumatology Composite Response Index (ACR CRISS) are composite scores which include common and distinct parameters to assess disease activity and treatment response in patients with dcSSc, respectively: EUSTAR AI integrates patient reported skin worsening, modified Rodnan skin score (mRSS), presence of digital ulcerations and tendon friction rubs, C-reactive protein and diffusing capacity of the lung for CO (DLCO, % predicted). ACR-CRISS bases on changes of mRSS, forced vital capacity (FVC), patient and physician global assessment and Health Assessment- Questionnaire Disability Index (HAQ-DI). In addition, a Revised CRISS has been proposed, that assesses response as improvement in ≥ 2 core set measures (mRSS, patient and physician global assessments, HAQ-DI ≥ 25%, 50%, and 75% or ≥ 5% for the FVC%) and no worsening in >1 core set measures.
Objectives: The aim of this study is to describe the trajectories of the revised ((EUSTAR)-AI), the ACR CRISS and Revised CRISS in a cohort of patients with dcSSc before and after CD19-CAR T-cell treatment at a single center.
Methods: Since April 20 th 2022, 12 patients with dcSSc were treated with CD19-CAR T-cells at the University Hospital Erlangen. 4 patients were treated as part of named patient use, and 8 patients were treated within the CASTLE study [3], the results of which are reported separately. All patients received an intravenous infusion of 1x10[6] CD19-CAR T cells/kg body weight, manufactured by using a lentivirus encoding a second generation CD19-CAR based on 4-1BB co-stimulation domain, after lymphodepletion with fludarabine (25mg/m 2 days -5 to -3 and cyclophosphamide 1g/m 2 i.v. day -3) [1, 2]. Parameters assessed included the EUSTAR AI as described by Valentini et al [4], ACR CRISS according to Khanna D, et. al [5] and revised CRISS as described by Khanna et al [6]. Spaghetti plots were drawn for EUSTAR-AI over time, ACR CRISS-score over time, and first time met revised CRISS by threshold, with a locally estimated scatterplot smoothing (LOESS) curve added to each plot. Median with interquartile range (IQR), or count with percentage were reported for clinical outcomes as appropriate. Missing data were imputed based on available data from consecutive visits.
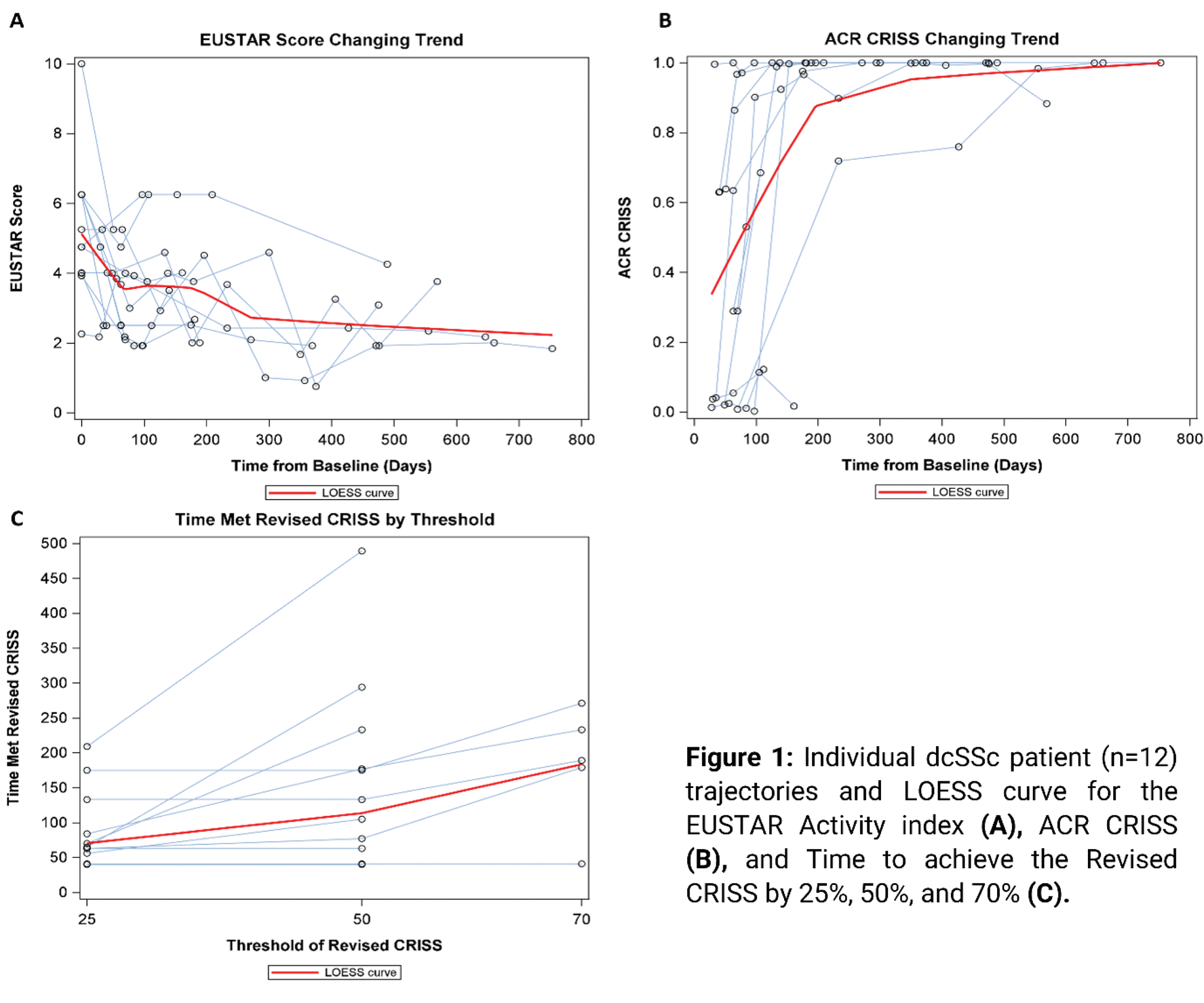
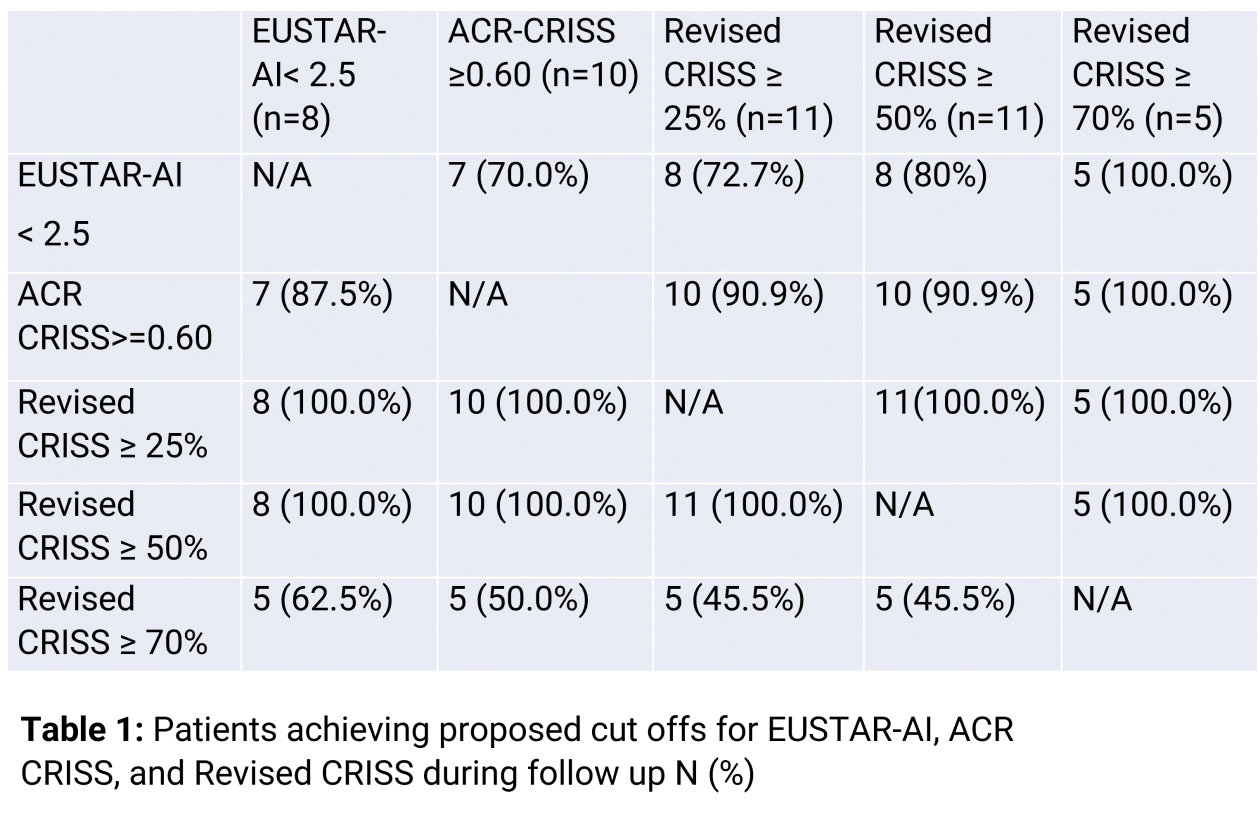
Results: Median follow up of all patients was 279 days (IQR: 136.5-529). The development of EUSTAR-AI and ACR-CRISS in all patients is visualized in Figures 1A and B and their central tendencies are approximated by LOESS curves. The median baseline EUSTAR-AI score was 5.0 (IQR: 4.0, 6.3) and 2.6 (IQR: 2.1-3.9) at last available visit. Eight patients (66.7%) achieved EUSTAR-AI < 2.5 at median of 211 days (IQR: 69.5-282.5) (Figure 1A). The strongest reduction of the EUSTAR-AI as summarized by LOESS curves was observed between day 0 and 69 and was 1.6 points. After this turning point, EUSTAR-AI LOESS curve further reduced with a smaller slope towards an asymptote of 2.0. Median ACR-CRISS at the last available visit was 1.00 (IQR: 0.66-1.00) (Figure 1B). Ten patients (83.3%) achieved ACR CRISS ≥ 0.6 at median of 80.5 days (IQR: 41-133). The steepest slope of ACR CRISS LOESS curve was observed between days 0 and 196, with an increase by 0.64. Revised CRISS with a 25% and 50% threshold was reached by 11 of 12 patients (91.7%), respectively, with a median time to achieve Revised CRISS 25% of 65 days (IQR: 56-133) and Revised CRISS 50% of 133 days (IQR: 63-233). Revised CRISS 70% was reached by 5 patients (41.7%) at a median of 189 days (IQR: 179-233, Figure 1C). respectively. EUSTAR-AI and ACR CRISS showed low correlation (-0.296; p=0.0228), highlighting that both measures complement each other. Median EUSTAR-AI at the time of the achievement of Revised CRISS 25%, 50% and 70% was 4.0 (IQR: 2.5-4.8), 3.0 (IQR: 2.4-4.3) and 3.7 (IQR: 2.1-3.8), respectively (table 1). 8 out of 11 (72.7%) patients who reached Revised CRISS of 25% and 50% and 5 out of 5 (100%) patients who reached Revised CRISS of 70% also reached EUSTAR AI below 2.5 points during the observational time. One patient did not reach any of the revised CRISS thresholds, however, this patient reached a EUSTAR-AI of 2.5 at 63 days, which is at least partly explained by improvement of CRP. Vice versa, out of 4 patients who did not reach EUSTAR-AI below 2.5 during follow up, 3 (75.0%), 3 (75.0%), and 0 (0.0%) patients reached revised CRISS 25%, 50% and 70%, respectively.
Conclusion: The majority of dcSSc patients with CD19-CAR T-cell treatment improve with both EUSTAR AI and ACR CRISS scores within the median observational time of 279 days. Median time to achieve EUSTAR < 2.5 and ACR CRISS ≥ 0.6 is 211 days and 80.5 days, respectively. However, both scores also show distinct patterns and complement each other.
REFERENCES: [1] Auth J, Müller F, et al. Lancet Rheumatol 2024.
[2] Müller F, Taubmann J, et al. N Engl J Med 2024;390:687-700.
[3] Schett G, Bohr D, et al. POS0030 Ann Rheum Dis 2024;83:327.
[4] Valentini G, et al. Ann Rheum Dis 2017;76:270-6.
[5] Khanna D, et al. Arthritis Rheumatol 2016;68:299-311.
[6] Khanna D, Huang S, et al Rheum Dis 2021;80:641-50.
Acknowledgements: NIL.
Disclosure of Interests: Christina Bergmann Novartis Argentina, Janssen, Boehringer-Ingelheim, Suiyuan Huang: None declared, Janina Auth Kyverna, Andreas Wirsching: None declared, Melanie Hagen: None declared, Havva Nur Kartalczik: None declared, Ricardo Grieshaber-Bouyer: None declared, Fabian Müller AstraZeneca, Abbvie, Beigene, BMS, Janssen, Kite/Gilead, Miltenyi, Novartis, Sobi, Taketa, AstraZeneca, BMS, CRISPR Therapeutics, Janssen, Kite/Gilead, Miltenyi, Novartis, Sobi, MedImmune/AstraZeneca, Kite/Gilead, BMS, Andreas Mackensen: None declared, Georg Schett: None declared, Dinesh Khanna Abbvie, Amgen, BMS, Astra Zeneca, Cabaletta, Fate Therapeutics, NKarta, Abbvie, Amgen, BMS, Astra Zeneca, Cabaletta, Fate Therapeutics, NKarta, Abbvie, Amgen, BMS, Astra Zeneca, Cabaletta, Fate Therapeutics, NKarta.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (