

Background: The STRATA-FIT consortium, a European Union funded research project, focuses on personalising management strategies for Difficult-To-Treat Rheumatoid Arthritis (D2T RA). It aims to develop and validate computational models that can help identify and group patients with D2T RA into relevant categories based on real-world clinical data. The STRATA-FIT Patient Advisory Panel, facilitated by EULAR, plays a pivotal role in overseeing project progress and providing input across all project phases. Patient involvement is central to the project, both for the success of its research objectives and for achieving the primary goal of delivering long-term benefits to patients through shared decision-making. However, several research projects nowadays involve patients as research partners (PRPs) without ever evaluating the quality of the collaboration and adapting engagement strategies, which can lead to tokenism, waste of resources and mismatched expectations.
Objectives: To evaluate the perspectives of both PRPs and researchers on the collaboration during the initial phase of the project. Additionally, we aim to assess which 2023 EULAR recommendations for the involvement of PRPs in rheumatology research have been successfully implemented, identify any gaps, and determine whether any adjustments or improvements are needed for the future.
Methods: After 6 months of collaboration, a 17-question survey was developed for a preliminary evaluation of the PRP engagement process and shared with the PRPs participating in STRATA-FIT.A full evaluation is now being conducted after 12 months of collaboration, including one 39-question survey for PRPs and one for researchers with the goal of collecting insights into the needs of both PRPs and researchers to improve the collaboration. Both surveys were adapted from the well-established Public and Patient Engagement Evaluation Tool (PPEET) and aligned with the respective 2023 EULAR recommendations.The questions for PRPs focused on topics such as: the perspective PRPs bring to the project, the frequency of interactions with researchers, the perceived level of collaboration within the project, whether they have opportunity to share their ideas and opinions, among others. The questions for researchers included: whether they worked with PRPs before this project, any challenges faced in collaborating with PRPs, whether they felt adequately knowledgeable to carry out their role and responsibilities toward the PRPs, among others. After this step, a list of key action points to improve the quality of the collaboration will be developed to be implemented by the consortium members.
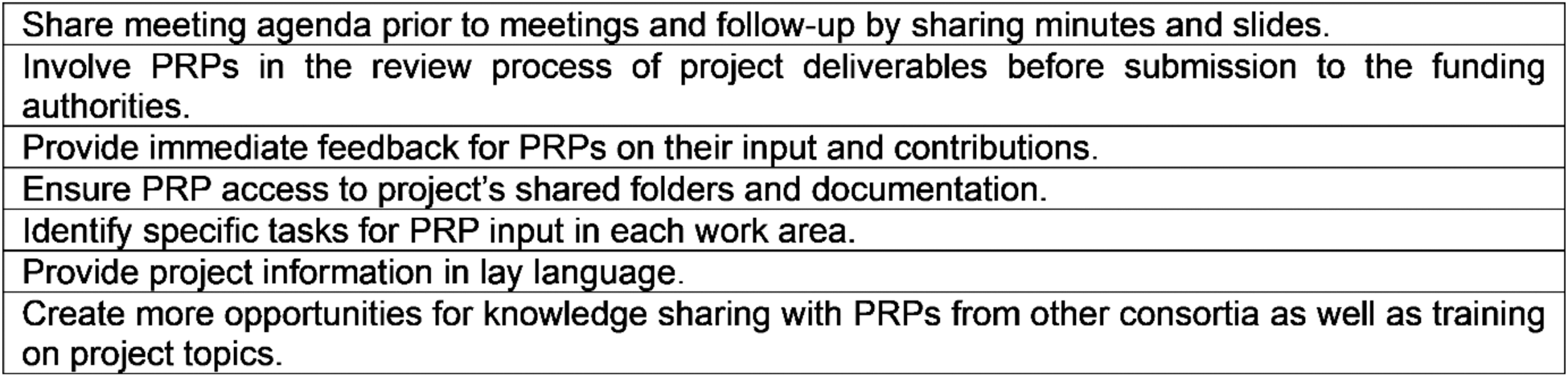
Results: The results of the preliminary evaluation showed that the six participating PRPs are overall satisfied with the STRATA-FIT patient engagement, assigning it an average rating of 4.6 out of 5. In this context, the following strengths of the patient involvement process were identified: support and communication provided by the EULAR Research team as PRP facilitator; possibility of PRPs giving input and influencing the project, which can potentially improve the lives of many patients; experience and knowledge learned by participating in such a large project; possibility of exchanging views and working together as a patient panel; diversity of PRPs regarding nationality, educational and professional background and experiences; discussions and skill share sessions with EULAR PRPs from other consortia. The following weaknesses of the process were also identified: lack of project information in lay language (both written and in meetings); amount of technical information often overwhelming; uncertainty about role of PRPs in meetings; uncertainty about tasks for PRPs to contribute to; lack of awareness of progress from other project areas; lack of follow-up information on meetings. Regarding the EULAR recommendations, five recommendations have been found to be fully implemented while the remaining five are considered partially implemented (see Table 1). After evaluating the strengths and weaknesses of the collaboration, the PRPs reached a consensus on the prioritization of improvement opportunities, and identified seven key action points for the future (see Table 2).
Table 1. List of the 2023 EULAR recommendations for the involvement of PRPs in rheumatology research and their level of implementation in the STRATA-FIT consortium.
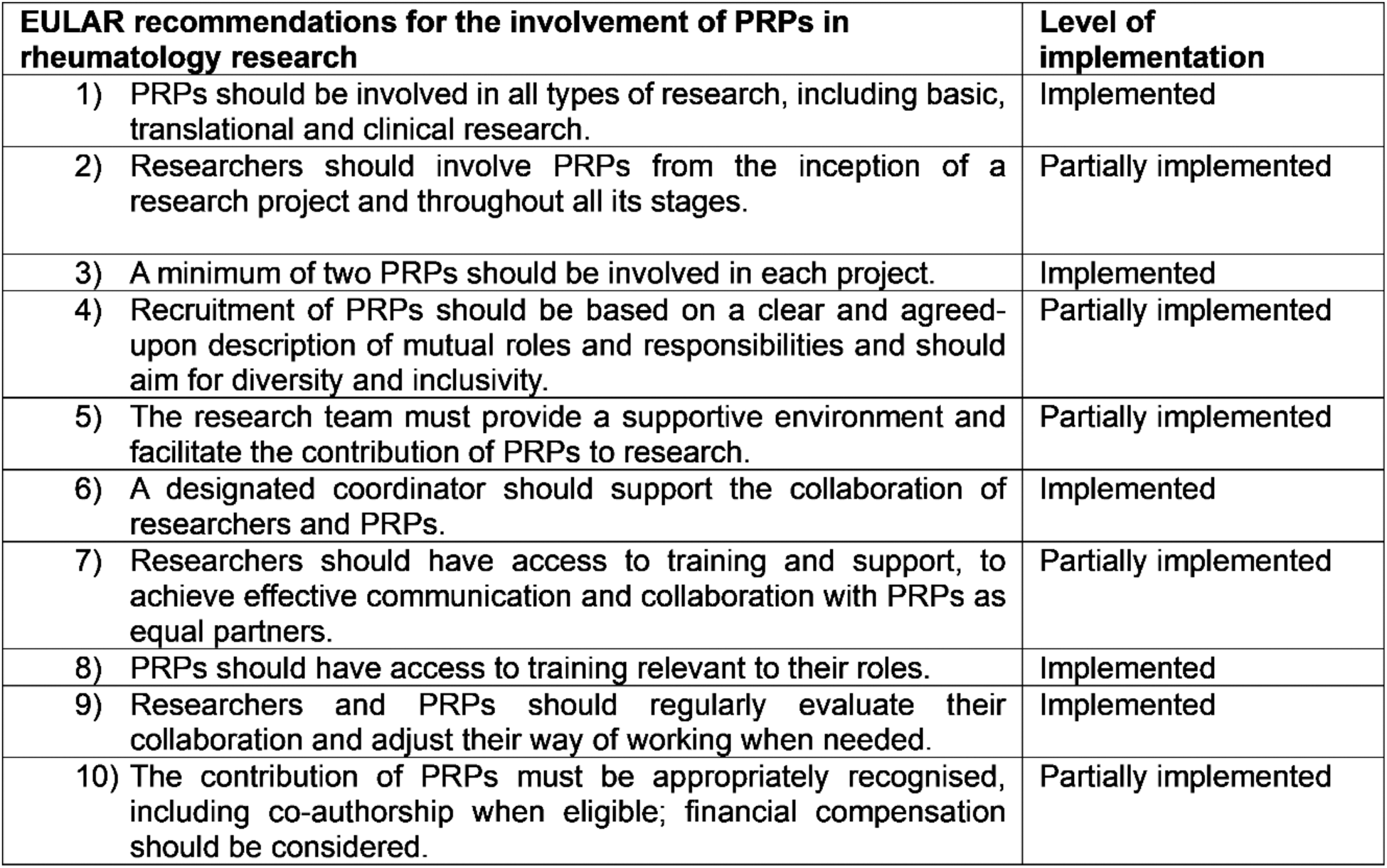
Table 2. List of seven consensually agreed preliminary action points for implementation in STRATA-FIT.
The analysis of responses from the full evaluation is currently ongoing and its results will iterate our action points.
Conclusion: The 6-month evaluation of PRP involvement in the consortium received positive feedback but still prompted the phrasing of key action points for future activities. They should strengthen the areas of inclusiveness and communication, emphasizing the need for sustained patient engagement and the integration of patient feedback into all phases of the work plan. The PRP facilitator and the leading researchers will be responsible for implementing these action points within the next 12 months of the project. These efforts will ensure that STRATA-FIT continues to meet its objectives in a patient-centered manner. Hopefully, the STRATA-FIT experience can also inspire and advise future research consortia on how to establish effective and meaningful collaboration between PRPs and researchers.
REFERENCES: NIL.
Acknowledgements: This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement no. 101080243, from the Swiss State Secretariat for Education, Research and Innovation (SERI) and from Hungary’s National Research, Development and Innovation (NRDI) Fund. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
Disclosure of Interests: Slađana Rumpl Tunjić: None declared, Diana Rodrigues: None declared, Bertha Maat: None declared, Sofia Valpereiro: None declared, Natasha Trehan AbbVie, UCB, AbbVie, Pfizer, Janssen, Organon, Birgit Barten: None declared, IRENE Pitsillidou: None declared, Elsa Mateus: None declared, Kristina Chingov: None declared, Paul Studenic AbbVie, AbbVie, Paco M.J. Welsing: None declared, Jacob M. van Laar Abbvie, Astra Zeneca, Alfasigma, Boehringer Ingelheim, GSK, Novartis, I have received research grants from Boehringer Ingelheim and Alfasigma.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (