

Background: IgG4-related disease (IgG4-RD) is a progressive, systemic, fibroinflammatory disease characterized by unpredictable and recurring flares, leading to organ damage and decreased quality of life. Inebilizumab is a humanized, glycoengineered, CD19-directed, monoclonal antibody that depletes B cells, including plasmablasts and some plasma cells, effectively in a targeted manner. MITIGATE (NCT04540497) is an international, randomized, blinded, placebo (PBO)-controlled Phase 3 trial that evaluates the safety and efficacy of inebilizumab as treatment for IgG4-RD.
Objectives: To evaluate the time of onset and durability of efficacy of inebilizumab in the MITIGATE trial.
Methods: Primary results of the MITIGATE trial have been previously reported (Stone et al, 2024). The primary endpoint (time to first adjudication committee (AC)-determined and investigator-treated IgG4-RD flare during the 52-week RCP) and all key secondary endpoints (annualised flare rate (AFR) during the RCP and the proportion of participants achieving flare-free, treatment-free complete remission or corticosteroid-free complete remission at Week 52) were met. Participants who completed the RCP and otherwise remained eligible had an opportunity to participate in an optional 3-year open-label period (OLP) during which all participants receive inebilizumab. Post hoc analyses were performed to assess the timing of onset of efficacy during the RCP and to explore the benefit of inebilizumab during the OLP.
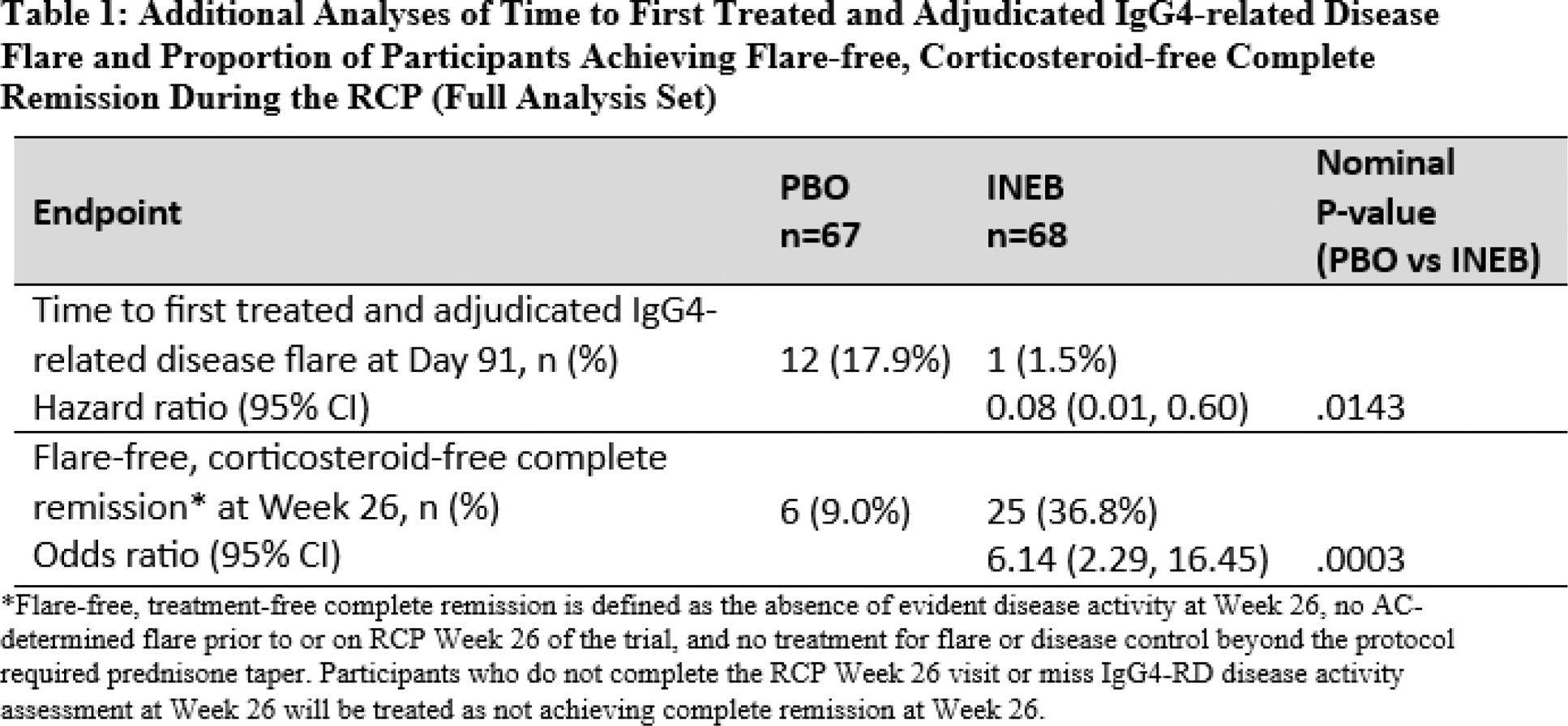
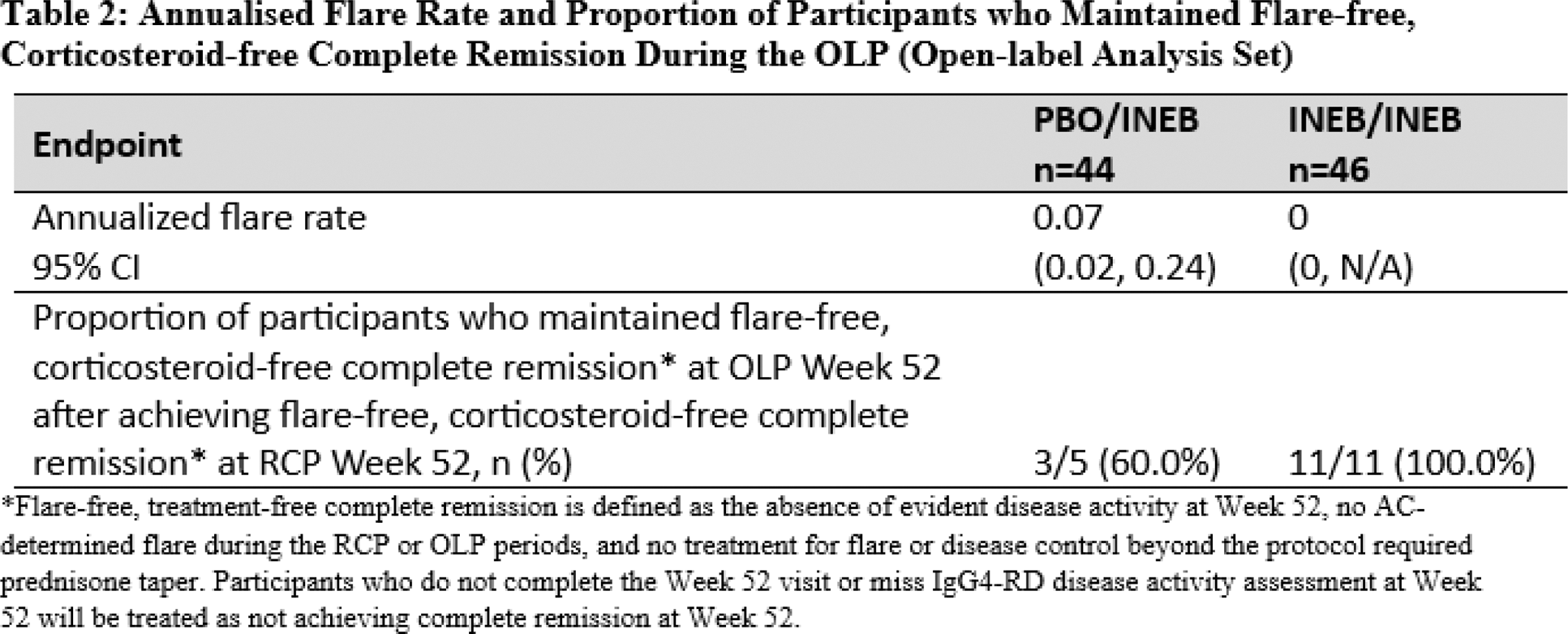
Results: 135 participants were randomised and received at least one dose of inebilizumab (n=68) or PBO (n=67) during the RCP. Statistically significant reduction in IgG4-RD flare risk of inebilizumab compared to PBO was evident as early as study day 91 (HR 0.08; 95% CI: 0.01, 0.60; nominal p=.0143), and statistically significant benefit continued through the end of the RCP. Similarly, by day 91 of the RCP, there was a significant difference in the proportion of participants who experienced an AC-determined and investigator-treated IgG4-RD flare (odds ratio 0.07; 95% CI: 0.01, 0.54; nominal p=.0111), which was also maintained at subsequent follow-ups during the RCP. Flare-free corticosteroid-free complete remission was achieved at RCP week 26 in a significantly higher proportion of participants in the inebilizumab arm than in the placebo arm (odds ratio 6.14; nominal p=.0003; Table 1). At the time of the primary analysis, 90 participants had received inebilizumab in the OLP (44 from the placebo arm and 46 from the inebilizumab arm). Exposure to inebilizumab during the OLP ranged from 90 to 688 days, with participants receiving 1 to 5 administrations of inebilizumab. The mean annualized flare rate for participants first receiving inebilizumab in the OLP was 0.07, similar to the AFR for the inebilizumab group in the RCP. No participant who received inebilizumab in the RCP and continued to receive inebilizumab during the OLP experienced a flare in the OLP. Among the 11 participants on inebilizumab who had achieved flare-free corticosteroid-free complete remission at RCP week 52 and continued to receive inebilizumab in the OLP, 100% maintained flare-free corticosteroid-free complete remission at OLP week 52 (Table 2). Furthermore, only one participant continuing inebilizumab in the OLP required glucocorticoid treatment for disease control during the OLP.
Conclusion: Analysis of MITIGATE trial data demonstrates that inebilizumab treatment provides significant benefit as soon as 91 days following treatment initiation, and continues to provide benefit during the second year of treatment, in patients with IgG4-RD.
REFERENCES: NIL.
Acknowledgements: Study and abstract were funded by Amgen Inc. with medical writing support provided by Ayven Davoudi, PharmD, Amgen Inc.
Disclosure of Interests: John H. Stone Amgen; argenx; Bristol-Myers Squibb; F. Hoffman-La Roche; IgG4ward! Foundation; Novartis; Q32 Bio; Sanofi; Sobi; Steritas; Zenas, National Institutes of Health, Emma L. Culver Amgen; Zenus, NIHR Oxford BRC, Arezou Khosroshahi Amgen, Wen Zhang Amgen, Kazuichi Okazaki Medpace, Yoshiya Tanaka Abbvie; Eisai; Chugai; Eli-Lilly; Behringer-Ingelheim; GlaxoSmithKline; Taisho; Astrazeneca; Daiichi-Sankyo; Gilead; Pfizer; UCB; Asahi-kasei; Astellas, Behringer-Ingelheim; Taisho; Chugai, Matthias Lohr: None declared, Nicolas Schleinitz Amgen, Xinxin Dong Amgen, Amgen, Betina Hernandez Amgen, Amgen, Sue Cheng Amgen, Amgen, Daniel Cimbora Amgen, Amgen.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (