

Background: Melanocortin receptor (MCR) agonists, such as Adrenocorticotropic hormone (ACTH), are endogenous pro-resolving mediators with pleotropic functions. The pharmacologic use of these agonists may act by triggering existing protective mechanisms to reduce inflammation. The active initial and cascading effects of these agonists may avoid additional tissue damage that often exacerbate inflammation induced bone loss.
Objectives: We aimed to evaluate the effects of a repository corticotropin injection (RCI; ANI Pharmaceuticals, Inc.), a complex mixture of ACTH, ACTH related peptides and other porcine pituitary derived peptides, when used as monotherapy in the mouse collagen-induced arthritis (CIA) model on the inflammatory response and bone damage.
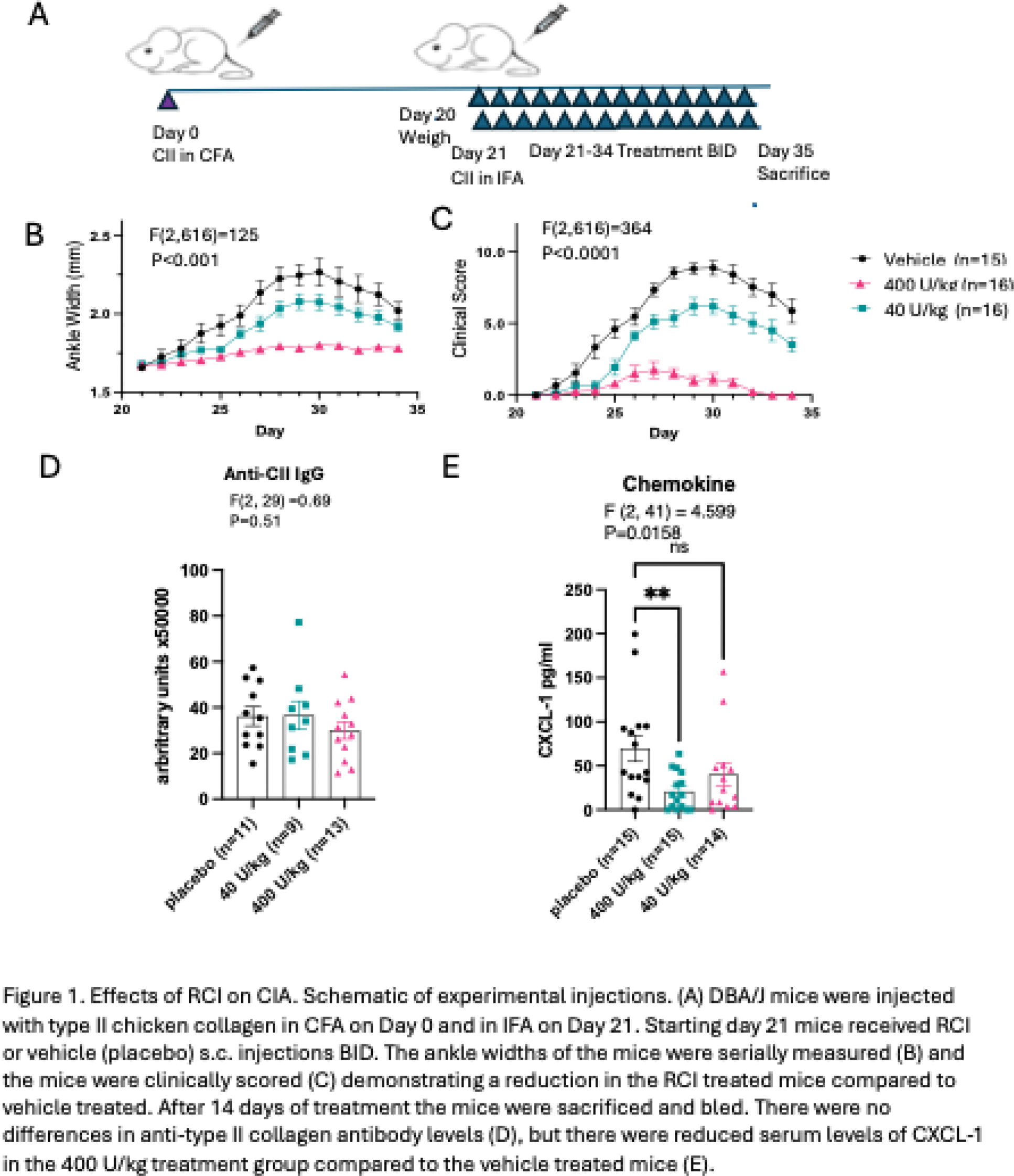
Methods: CIA was induced in six-week old male DBA/1J mice (IMSR_JAX:000670; Jackson Lab) by subcutaneous injection with complete Freund’s adjuvant (7001, Chondrex, USA) emulsified with type II collagen (20011, Chondrex, USA) at a ratio of 1:1, and incomplete Freund’s adjuvant (7002, Chondrex, USA) and type II collagen on Day 21. After induction of CIA, mice (n = 15 per group) received subcutaneous RCI (40, or 400 U/kg twice daily), or vehicle days 21 to 35 (Figure 1A). Inflammation was assessed via changes in hind paw thickness measured with a caliper and joint scores (28-point scale) and area under the curve (AUC) calculated. Hind limbs (day 35) were imaged with a micro-computed tomography (CT) scanner, Skyscan 1076 (Kontich, Belgium) at 9μm voxel size by standardized methods. Cortical bone analysis was performed at the femoral diaphysis in a 900 μm region, 4,500-5,400 μm proximal to the distal femoral growth plate. Trabecular bone analysis was performed at the distal femoral metaphysis in a 900 μm region, 450-1,350 μm proximal to the distal growth plate. Parameters determined by microCT included bone volume/tissue volume (BV/TV), bone mineral density (BMD), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp). CXCL-1 (KC) and anti-mouse CII antibody levels in sera were determined by ELISA (R&D Systems and Chondrex). Statistical analyses were performed using a 2-way analysis of variance (ANOVA) followed by the Bonferonni multiple comparisons test or a 1-way ANOVA followed by the Dunnett’s or Holm-Sidak multiple comparisons test.
Results: RCI administration resulted in dose-dependent decreases in ankle swelling in the 40, or 400 U/kg treatment groups compared to vehicle treated [AUC 2.9±0.39, 1.0±0.19, and 5±0.7, respectively; F(13, 616)=24, P<0.001; Figure 1B] and similarly for clinical scores [AUC 47 ±5.3, 9.4±3.2 and 75±5.9; F(13,616)=48.59; P<0.0001; Figure 1C]. There were no differences in the levels of anti-mouse type II collagen antibody generation between the placebo, 40, or 400 U/kg treatment groups (72377±8420, 73167±12348, 59819±7555 arbitrary units, respectively; P=0.51; Figure 1D). There were lower levels of serum CXCL-1 in the 400 U/kg treatment compared to the placebo treated group [20.92±5.5 pg/ml and 70±14.9 pg/ml respectively; F (2, 41) = 4.599; P=0.016; Figure 1E]. There were no differences in BV/TV, BMD, Tb.Th., and Tb.Sp. as assessed by microCT between groups.
Conclusion: As monotherapy, RCI significantly attenuated CIA-induced joint swelling in mice. In addition, RCI treatment resulted in a reduction in inflammatory cytokine response, but no disruption of IgG formation and no additive bone loss. These findings demonstrate significant reductions in systemic inflammation in the CIA arthritis model.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Maripat Corr received lab service agreement from ANI Pharmaceuticals, Inc., Aaron Place is an employee of ANI Pharmaceuticals, Inc., Grace Hsueh is an employee of ANI Pharmaceuticals, Inc., Nancy Lane is a paid consultant for ANI Pharmaceuticals, Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (