

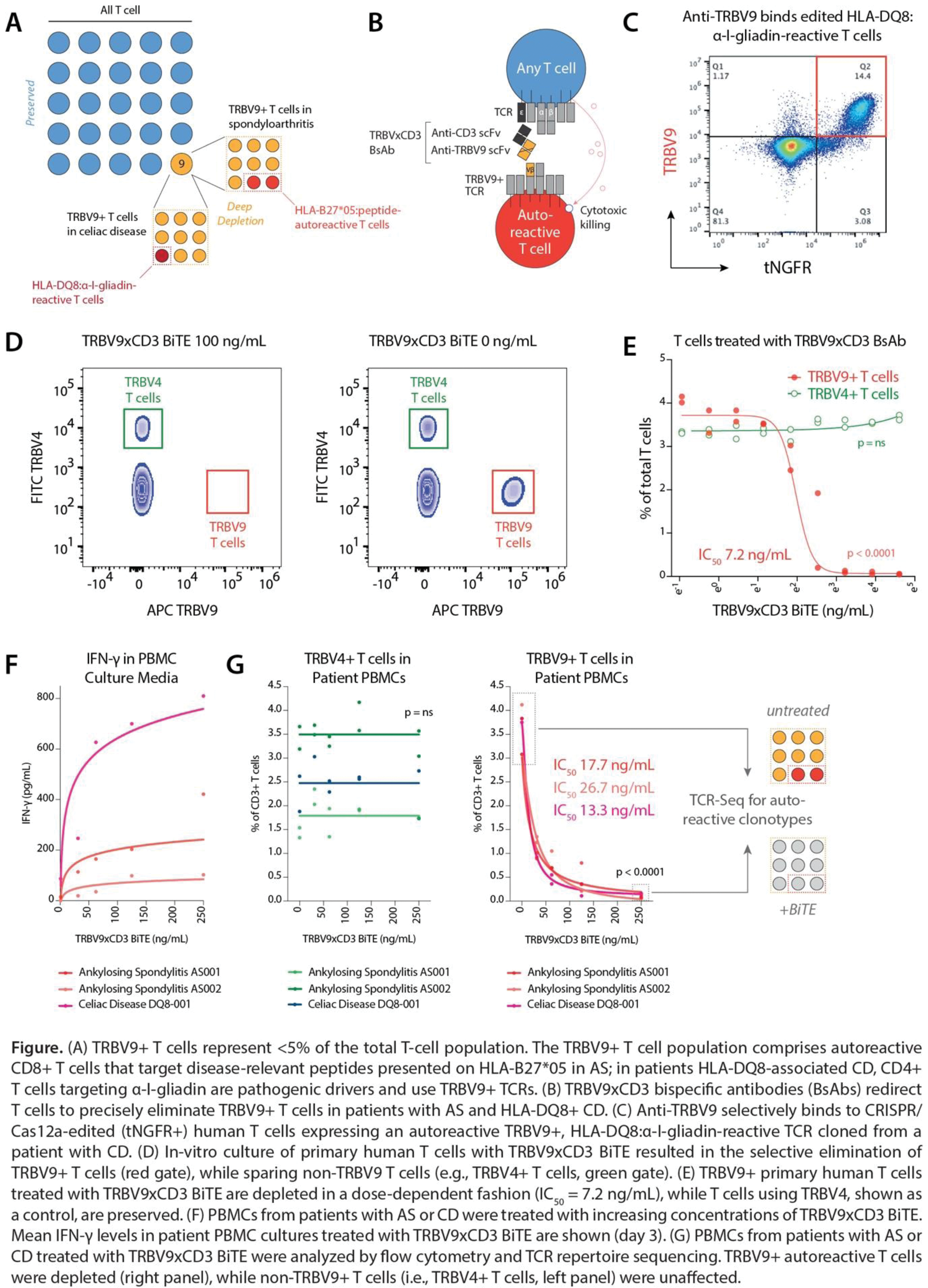
Background: Therapies that indiscriminately deplete all T cells carry a high risk of opportunistic infection, precluding their widespread use in T cell-mediated autoimmune diseases. Targeting autoreactive T cells with selectivity, conversely, is difficult given an incomplete understanding of their antigen specificities. Germline-encoded T cell receptor (TCR) regions provide an opportunity to target autoreactive T cells, even if the cognate autoantigen is unknown. TCR β-chain variable (TRBV) gene usage by autoreactive T cells can be highly skewed by HLA alleles. In axial spondyloarthritis (AS), CD8+ T cells against disease-relevant peptides presented on HLA-B27 are contained in the TRBV9+ T cell fraction. Analogously, HLA-DQ8:α-I-gliadin-reactive CD4+ T cells that drive celiac disease (CD) use TRBV9 (A). Deep depletion of the TRBV9+ T cell compartment has the potential to achieve long-standing disease remission in patients with AS and CD without increasing the risk of infection.
Objectives: We aimed to develop a precision T cell-engaging bispecific antibody (BsAb) therapy that selectively eliminates TRBV9+ T cells in AS and CD, while sparing >95% of the T cell repertoire.
Methods: Anti-TRBV9 and anti-CD3 single-chain variable fragment (scFv) sequences were synthesized, cloned, and TRBV9xCD3 BsAbs expressed as single-chain diabodies (scDbs) and bispecific T-cell engagers (BiTEs). Human T cells were CRISPR-Cas12a-edited to replace their endogenous TCRs with autoreactive, TRBV9+ TCRs cloned from patients with AS or CD. Binding of anti-TRBV9 to edited T cells was determined by flow cytometry. The potency and specificity of TRBV9xCD3 BsAbs against primary human T cells was interrogated in culture, and cytotoxicity was quantified by flow cytometry and interferon (IFN)-γ ELISA. PBMCs from patients with HLA-B27+ AS and HLA-DQ8+ CD were treated with BsAbs, and selective depletion of TRBV9+ T cells quantified by flow cytometry and TCR repertoire sequencing.
Results: We developed BsAbs to redirect T cells to selectively eliminate TRBV9+ T cells that harbor the autoreactive T cell compartment in patients with AS and HLA-DQ8+ CD (B). Anti-TRBV9 bound engineered human T cells expressing autoreactive TRBV9+ TCRs cloned from patients with AS or CD, but not T cells expressing other TRBV alleles (C). In culture of primary human T cells, TRBV9xCD3 BiTEs resulted in the selective elimination of TRBV9+ T cells in a dose-dependent manner (p< 0.0001), sparing T cells with other TRBV gene usage (e.g., TRBV4+ T cells) (p=ns) (D-E). Treatment of PBMCs from patients with AS and HLA-DQ8+ CD with TRBV9xCD3 BiTEs induced limited IFN-γ release (F) and depletion of TRBV9+ T cells, but not non-TRBV9 T cells (p=ns) (p< 0.0001) (G). TCR repertoire sequencing confirmed the elimination of disease-relevant autoreactive T cell clonotypes in AS patient PBMCs treated with TRBV9xCD3 BiTEs. Half-life extended (HLE) BsAbs showed comparable efficacy and specificity to non-HLE TRBV9xCD3 BiTEs.
Conclusion: We describe an off-the-shelf, precision immunotherapy approach for the deep depletion of autoreactive TRBV9+ T cells in HLA-B27+ AS and HLA-DQ8+ CD. TRBV9xCD3 BsAbs are highly potent and specific at eliminating TRBV9+ T cells while sparing other T cell populations. Different to monoclonal antibodies targeting TRBV9, BsAbs may offer an opportunity to achieve sustained depletion and reset of the autoreactive T cell compartment in AS and CD.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Stephanie Glavaris: None declared, Alexander Pearlman: None declared, Jin Liu: None declared, Jiaxin Ge: None declared, Yuanxuan Xia: None declared, Kyle K. Kaeo: None declared, Tolulope Awosika: None declared, Colin Gliech: None declared, Tushar Nichakawade: None declared, Nikita Marco: None declared, Chetan Bettegowda Belay Diagnostics (Ownership Interest), OrisDx (Ownership Interest), Bionaut Labs (Consultant), Depuy-Synthes (Consultant), Haystack Oncology (Consultant), Privo Technologies (Consultant), TBD Pharma (Consultant), Drew Pardoll BMS (Royalties), Potenza (Ownership Interest), TBD Pharma (Ownership Interest), Aduro Biotech (Consultant), Amgen (Consultant), Astra Zeneca (Consultant), Bayer (Consultant), Camden Nexus II (Advisor or Review Panel Member), DNAtrix (Consultant), Dracen Pharmaceuticals (Officer or Board Member), Dynavax Technologies Corporation (Consultant), Ervaxx (Consultant), Five Prime Therapeutics (Advisor or Review Panel Member, FLX Bio (Consultant), Immunomic Therapeutics (Consultant), Janssen, Merck (Consultant), Rock Springs Capital (Consultant), Tizona (Consultant), WindMil (Advisor or Review Panel Member), TBD Pharma (Consultant), Compugen (Grant/Research Support), Suman Paul Clasp (Intellectual Property/Patents, Royalties), TBD Pharma (Ownership Interest), Curio Science (Consultant), IQVIA (Consultant), Merck (Consultant), TBD Pharma (Consultant), Kenneth W. Kinzler CAGE Pharma (Ownership Interest), Clasp (Intellectual Property/Patents, Ownership Interest), Exact Sciences (Ownership Interest), Haystack Oncology (Intellectual Property/Patents, Ownership Interest), Neophore (Ownership Interest), Personal Genome Diagnostics (Ownership Interest), Thrive Earlier Detection (Intellectual Property/Patents, Ownership Interest), TBD Pharma (Ownership Interest), Clasp (Consultant), Neophore (Consultant), Personal Genome Diagnostics (Consultant), Thrive Earlier Detection (Consultant), TBD Pharma (Consultant), Shibin Zhou Clasp (Intellectual Property/Patents, Ownership Interest), Exact Sciences (Ownership Interest), Neophore (Ownership Interest), Personal Genome Diagnostics (Ownership Interest), TBD Pharma (Ownership Interest), Clasp (Consultant), Neophore (Consultant), Personal Genome Diagnostics (Consultant), TBD Pharma (Consultant), BioMed Valley Discoveries (Grant/Research Support), Bert Vogelstein Clasp Therapeutics (Intellectual Property/Patents, Ownership Interest), Haystack Oncology (Intellectual Property/Patents, Ownership Interest), Thrive Earlier Detection (Intellectual Property/Patents, Ownership Interest), TBD Pharma (Ownership Interest), Catalio Capital Management (Consultant), Clasp Therapeutics (Consultant), Haystack Oncology (Consultant), Thrive Earlier Detection (Consultant), TBD Pharma (Consultant), Maximilian F. Konig TBD Pharma (Ownership Interest), Allogene (Consultant, Scientific Advisory Board), Argenx (Consultant), Atara Biotherapeutics (Consultant), Bristol Myers Squibb (Speaker), Revel Pharmaceuticals (Consultant), Sana Biotechnology (Consultant, Scientific Advisory Board), Sanofi (Consultant), TBD Pharma (Consultant).
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (