

Background: RA-ILD has a high incidence, high mortality and short average survival time, but the disease and its pathogenesis are not clear. The clinical biological targeted therapy has limited efficacy, poor prognosis, and some have pulmonary toxicity and aggravation of the disease.
Objectives: The aim of this study is to establish Rheumatoid arthritis-associated Interstitial lung disease by verifying collagen-induced arthritis and bleomycin-induced interstitial lung disease disease,RA-ILD) mouse model, to explore the feasibility of the construction of a double-hit mouse model, and use this as a model animal, To explore the feasibility and effectiveness of using Mesenchymal stem cells (MSCs) -derived Exosomes (MSCs-Exo) to package soluble vascular endothelial growth factor receptor 1 (Sflt-1) gene to alleviate joint and lung inflammatory injury in model mice. The mechanism by which MSCs-derived Sflt-1 Exo alleviates arthritis and lung inflammation by antagonizing angiogenesis in mice was analyzed from macroscopic, pathological, imaging and inflammatory factors, which provides a basis for the targeted treatment of RA-ILD by this target.
Methods: (1) ADSCs were isolated from inguinal fat pad of 5 8-day-old C57B6 mice and cultured. The morphology of ADSCs was observed under a microscope, and the surface markers were identified by flow cytometry. (2) The Sflt-1 plasmid was constructed and the supernatant of ADSCs was collected after transfection. (3) ADSCs-Exos were extracted, and their morphological characteristics were observed by transmission electron microscopy. Nanoparticle tracking analysis (NTA) was used to analyze the particle size of ADSCs-Exos. (4) Twenty-four male DBA-1 mice were randomly divided into control group (Con group), collagen combined with bleomycin induced Model group (Model group), and RA-ILD model group treated with exosomes packed with Sflt-1 (RA-ILD+Sflt-1) Exo group) and RA-ILD model mice treated with empty Sflt-1 exosomes (RA-ILD+ non-sFlt-1 Exo group). (5) The RA-ILD mouse model was established by tail intradermal injection of collagen combined with intratracheal instillation of bleomycin. The degree of paw swelling of the mice was observed (the changes of foot pad retrodegree, body weight and joint diameter of the mice were recorded every 4 days) and lung CT examination was performed. (6) After intratracheally instillation of BLM, 100ul of exosomes packaged with Sflt-1 and exosomes unloaded with Sflt-1 were injected via tail vein to evaluate the therapeutic effect. At the end of the experiment, the mice were sacrificed, and pathological sections of joint and lung tissues were stained to compare the pathological changes. (7) Primers were designed using the NCBI database. The mrna expression levels of pro-inflammatory cytokines IL-1β, IL-6, IL-17A, TNF-α and anti-inflammatory cytokines IL-10 in joint and lung tissues and pro-fibrotic factors vcam, α-sma and TGF-β in lung tissues were detected
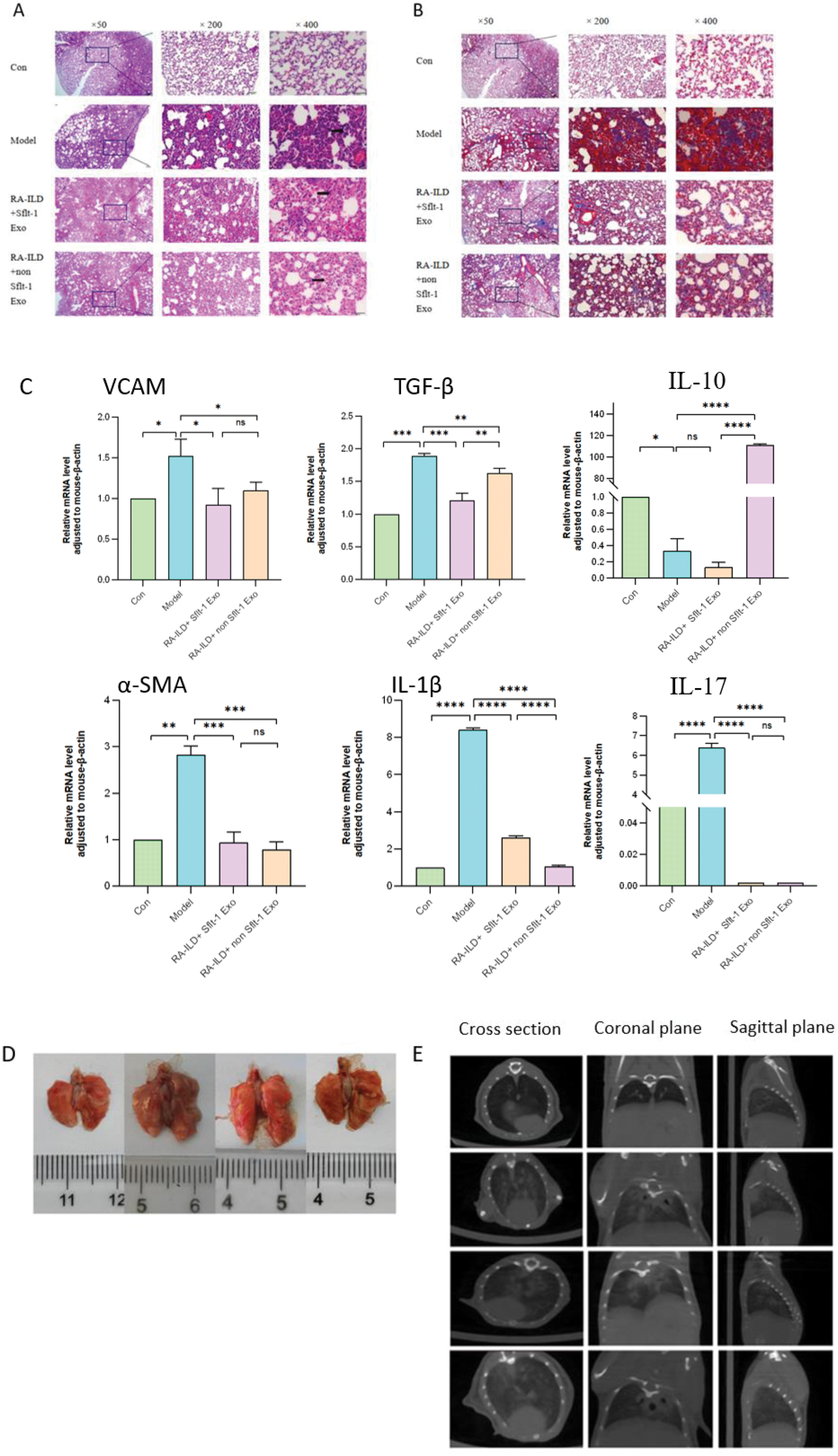
Results: (1) Sflt-1 plasmid and negative control were successfully transfected into ADSCs. RT-qPCR showed that there was a significant difference in the expression of Sflt-1 in ADSCs transfected with plasmid. (2) The concentration, particle size and surface markers of ADSCs-Exo were consistent with the general characteristics of Exo. Sflt-1 was highly expressed in Exo packed with Sflt-1. (3) The exosome intervention groups could alleviate joint inflammation, reduce the levels of IL-1β and IL-6, and improve the results of joint pathological sections. (4) In the exosome intervention group, the fibrosis level and pulmonary inflammation were improved, and the inflammation and collagen deposition in the lung pathological sections were reduced. Among them, the pro-fibrotic factors vcam and TGF-β were statistically significant between the RA-ILD+Sflt-1 Exo group and the RA-ILD+ non-sFlt-1 Exo group, and IL-17 and IL-6 were decreased after the intervention of different exosomes. IL-10 was significantly increased in RA-ILD+ non Sflt-1 Exo group (Figure 2 A, B, C). (5) The pulmonary ground glass opacities and fibrous cords were significantly improved in the exosome intervention group, and there were significant differences between the RA-ILD+Sflt-1 Exo group and the RA-ILD+ non-sFlt-1 Exo group (Figure 2 D, E).
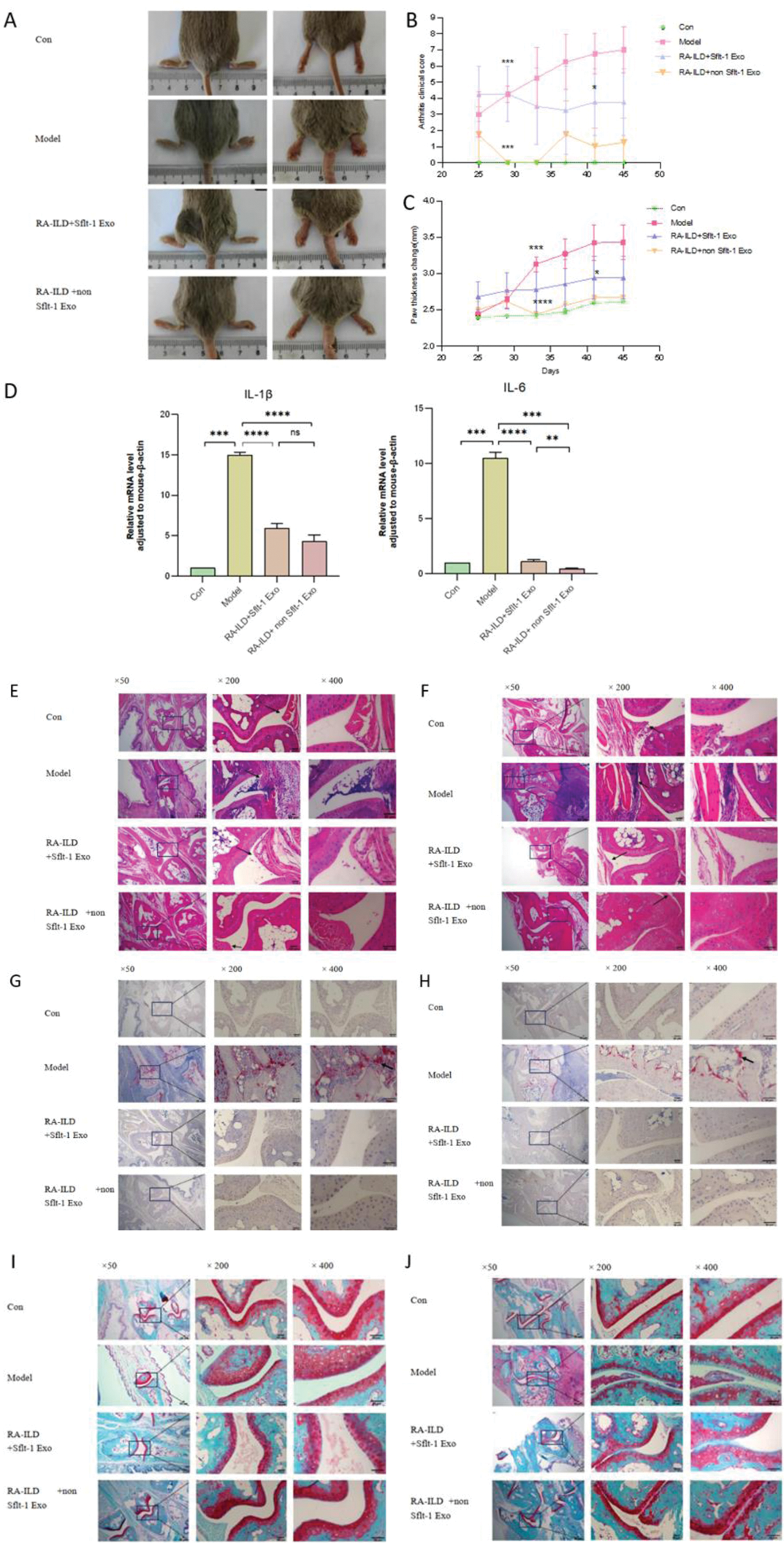
Joint changes in mice
A Comparison of the degree of joint swelling in the anteroposterior and lateral positions of mice; B Comparison of clinical arthritis scores in mice; C Changes in paw thickness of mice; D mRNA expression levels of joint inflammatory factors; E Proximal interphalangeal joints were stained with HE; F knee joint HE staining; G proximal interphalangeal joint Trap staining; H knee joint Trap staining; I Safranin fast green staining of proximal interphalangeal joints; J Knee joint was stained with safranin fast green
Pulmonary pathological, gross and imaging changes
A mice lung tissues were stained with HE; B Masson staining; C mRNA expression levels of inflammatory and fibrotic factors in lung tissues of mice;D Photos of lung tissue of mice sacrificed for sampling; E CT images of the lungs of mice 21 days after instillation of BLM.
Conclusion: Collagen combined with BLM can successfully construct a mouse model of RA-ILD. Intravenous injection of ADSCs-Exo can reduce arthritis, the degree of lung tissue fibrosis, the expression of inflammatory factors, fibrosis factors and pathological damage. The specific dosage of exosomes in the treatment of RA-ILD mice with targeted VEGF still needs to be adjusted in the future. ADSCs-Exo may become an effective drug for the treatment of RA-ILD with non-cellular therapy and an efficient drug carrier.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (