

Background: Sjögren’s disease (SjD) is a systemic autoimmune disease that is characterized by dryness and systemic manifestations. Salivary gland epithelial cells (SGEC) are pivotal in SjD pathogenesis, being both targets and active actors via interactions with immune cells. Current in vitro models fail to capture salivary glands complexity, underlining the need for innovative models that replicate epithelium/immune cell interactions.
Objectives: Our primary objective was to build upon our previous work on salivary gland organoids derived from labial salivary gland biopsies (LSGB), studying their development, differentiation, and detailed characterization. This included identifying and analyzing the distinct epithelial cell subtypes within differentiated salivary gland organoids (DSGO) from SjD patients and controls. To further progress our research, our secondary objective was to establish a more disease-relevant model by introducing B cells into the system. This innovation led to the development of immune-organoids (ISGO), providing a platform to investigate immune-epithelial interactions and their role in the pathogenesis of SjD.
Methods: Salivary gland organoids were generated from LSGB of SjD patients and controls. RNA sequencing (RNA-seq) was performed to further characterize DSGOs. Deconvolution analysis of RNA-seq data was a key focus, enabling the identification and detailed characterization of distinct epithelial subtypes within DSGOs. Organoid functionality was evaluated through swelling assays using live-cell imaging to measure their response to pilocarpine and assess drug efficacy. To develop immune-organoids (ISGOs), peripheral blood-B cells from blood donors were introduced to the system and were co-cultured with epithelial organoids in ULA 96-well plates. B cell viability and activation were assessed by flow cytometry after five days of co-culture. Comparative swelling assays were conducted on immune-organoids and epithelial organoids.
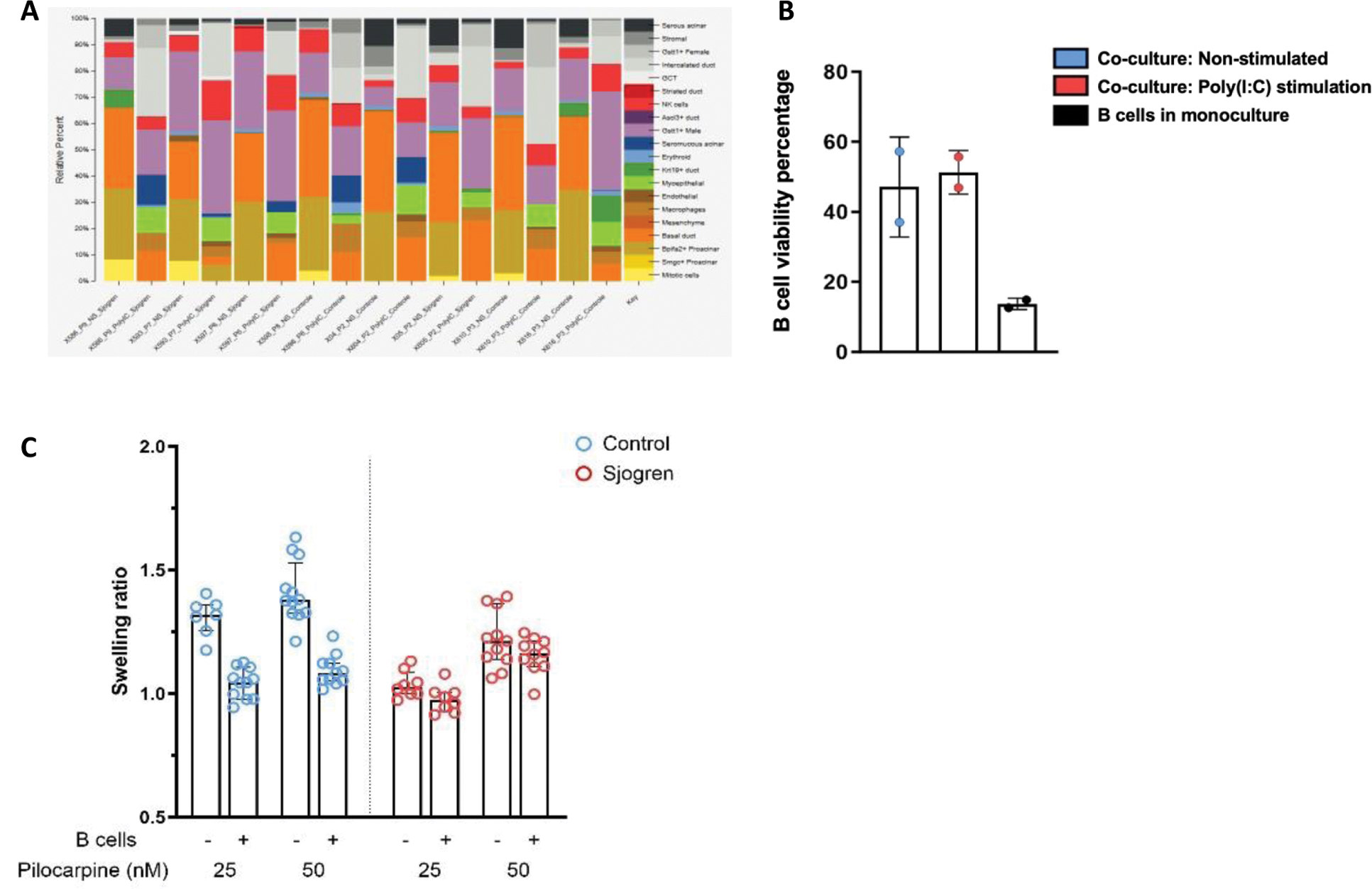
Results: Deconvolution analysis of RNA-seq data provided insights into the cellular heterogeneity of DSGOs, revealing the presence of distinct ductal and acinar cell subtypes in organoids derived from both controls and SjD patients. The analysis identified variations in epithelial cell populations, including the enrichment of specific acinar markers associated with salivary gland function and the differential expression of ductal markers between the two groups (Figure 1A). In early trials to develop immune-organoids (ISGOs), peripheral blood-derived B cells were successfully co-cultured with DSGOs in ULA 96-well plates. B cell viability and activation were evaluated via flow cytometry after five days of co-culture, showing promising outcomes for the integration of immune cells into the organoid system (Figure 1B). Functional assessment using swelling assays revealed notable differences between DSGOs and ISGOs. DSGOs from control samples exhibited a robust swelling response when stimulated with pilocarpine, However, the introduction of B cells into control-derived DSGOs to generate ISGOs resulted in a reduction in swelling capacity, reflecting the impaired functionality seen in SjD-derived DSGOs. (Figure 1C).
Characterization of B Cell–Salivary Organoid Co-Culture . A) Convolution analysis of RNA sequencing data reveals distinct epithelial cell populations within salivary gland organoids. B) Percentage of viable B cells in co-culture with salivary gland organoids without stimulation and following stimulation with Poly (I:C) as well as B cells cultured alone. C) Swelling assay results following 24-hour exposure of organoids and immune-organoids to pilocarpine, n=number of replicates per condition (n > 7).
Conclusion: Salivary gland organoids are an interesting model for studying the pathophysiology of SjD. The model developed here incorporates a diversity of epithelial subtypes, thus reproducing the complexity of the epithelial environment in patients. To advance the study of the disease, we are developing an immuno-organoid model. Initial experiments show that co-culturing B cells with the organoid promotes B cell survival. Adding B cells to the organoids reduces their swelling capacity. Complementary experiments are underway to decipher the interactions between the epithelium and B cells, their impact on secretory function, and potential therapeutic approaches.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (