

Background: Treatment of autoimmune disease with T cell engagers (TCEs) targeted against CD19 or BCMA leads to profound B cell depletion, followed by reconstitution [1, 2]. However, the dynamics of TCE activity in vivo, the dynamics of B cell depletion and reconstitution and the potential impacts on the endogenous T cell compartment in autoimmune disease are poorly understood.
Objectives: To investigate the effects of TCE therapy on the B cell and T cell compartment in patients with different autoimmune diseases (AIDs), including rheumatoid arthritis (RA), systemic sclerosis (SSc), idiopathic inflammatory myositis (IIM), IgG4-related disease (IgG4-RD) and Grave’s disease (GD).
Methods: 14 patients with RA were treated in our department with the TCE CD19xCD3 Blinatumomab, 10 patients with various autoimmune diseases including SSc, IIM, IgG4-RD and GD were treated with the plasma cell-targeting TCE BCMAxCD3 teclistamab. Peripheral blood mononuclear cells (PBMCs) were isolated from each patient at baseline and regularly after the treatment. High dimensional flow cytometry was performed on PBMCs to quantify T and B cell phenotypes. 1-year follow-up was available for 8/14 patients treated with blinatumomab. 6-months follow-up was available for 5/10 patients treated with teclistamab.
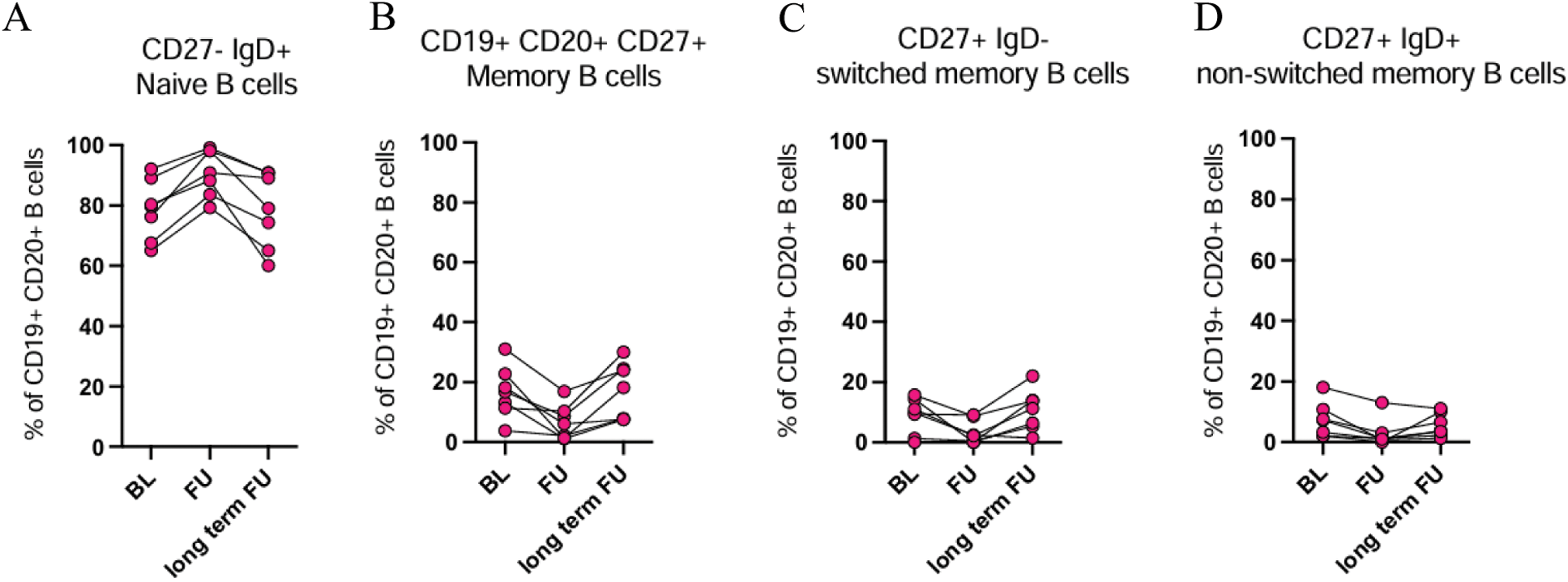
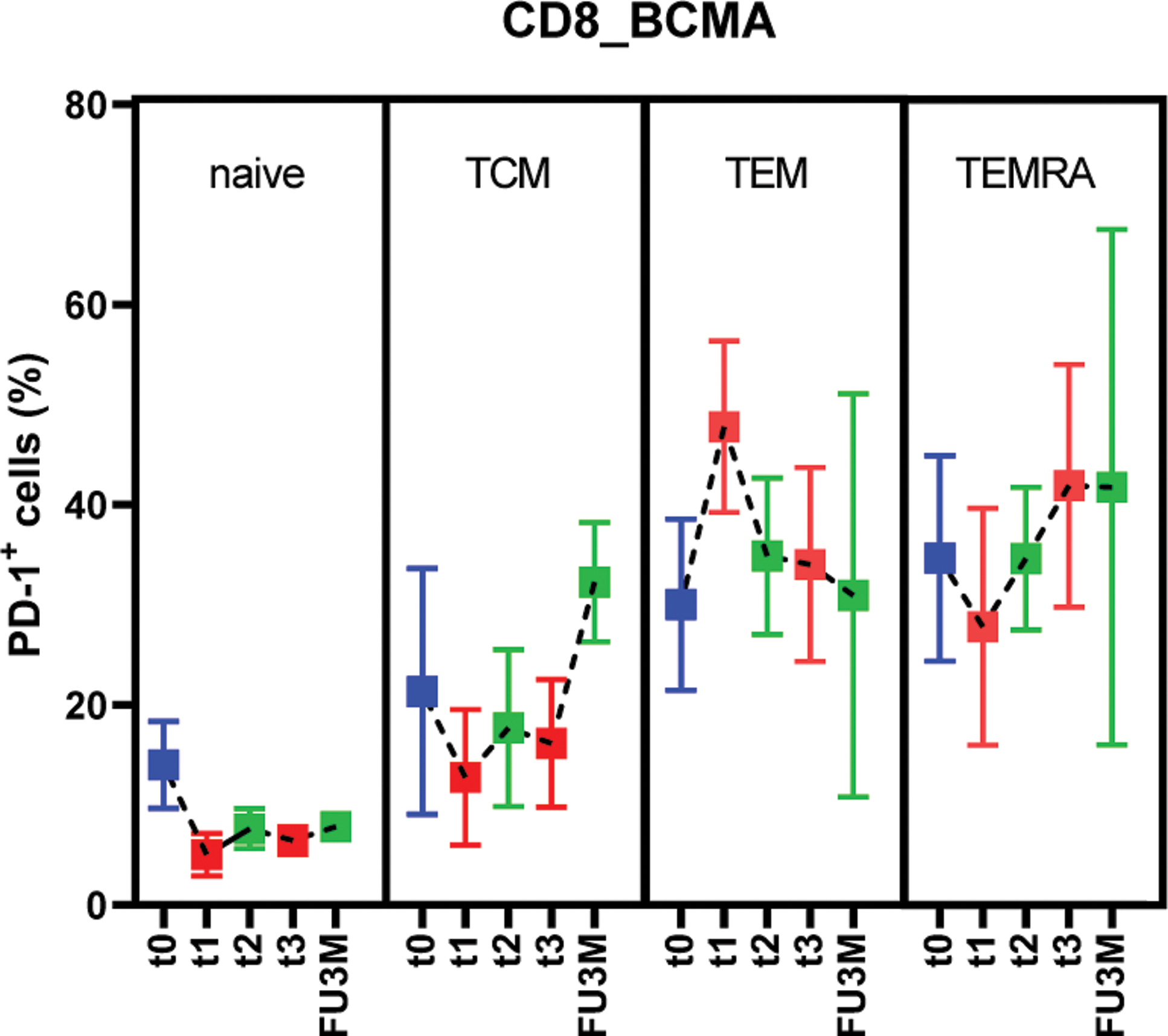
Results: Both blinatumomab and teclistamab lead to a rapid depletion of B cells in peripheral blood. 11/14 RA patients had circulating B cells at baseline (median 114/nl). In three patients, B cells were already peripherally depleted because of previous rituximab therapy (mean 3.3 months before blinatumomab [range: 3–4]). Blinatumomab lead to complete B cell depletion in 8 patients. B cell aplasia was reached after the first cycle in 2 patients and after the second cycle in 4 patients. 3/14 patients received a third cycle of blinatumomab, in 2 of them B cells were completely depleted after the third cycle. Further, 3 patients treated with blinatumomab did not have a complete B cell depletion. Teclistamab lead to complete B cell depletion in 10/10 patients. B cell aplasia was reached after the first teclistamab dose in 4/10 patients and in 6/10 patients after the third dose. B cells reconstituted after blinatumomab therapy after a median of 63 days (range 32-139) and after teclistamab after a median of 115 days (range 84-161). Reconstituted B cells were mostly of a naive B phenotype (mean value 84% in blinatumomab [range 66–90%], mean value 88% in teclistamab [range 85–96%]). Correspondingly, CD19 + CD27 + memory B cells decreased substantially, in blinatumomab from an average of 16%, [range 6.3–31%] to 7%, [range 0–16%] and in teclistamab from an average of 8% [range 2.6–18%], to 2%, [range 0.5–3.2%]. At one year follow up after blinatumomab initiation (6/14 patients), we identified a reconstitution of both CD27 + IgD + non-switched and CD27 + IgD - switched memory B cells, while CD20 + CD27 - naive B cells slightly decreased to an average of 70% (Figure 1a, b, c, d). Plasmablasts were depleted after both therapies and stayed depleted at long-term follow up. Consistent with T cell engagement, TCE therapy lead to a robust increase in exhaustion related T cell phenotypes. In particular, after the first cycle of blinatumomab and after completion of teclistamab step up dosing, we observed an accumulation of PD1+ exhausted CD8 + T cells (blinatumomab: from 11.2± 5.8% to 20.7 ± 4.1%; teclistamab: from 6.9± 6.6% to 24.3± 9.2%). In addition, Teclistamab therapy lead to an increase in CD62L - CD45RA - CD8 + effector memory (EM) and CD62L - CD8 + effector memory re-expressing CD45RA (EMRA) T cells. CD8 + TEM and TEMRA also expressed more PD-1 compared to the other CD8 + subpopulations, suggesting increased exhaustion (Figure 2). An increase in CD8 + TEM was also observed after the first cycle of blinatumomab therapy.
Conclusion: Together, these data demonstrate the impact of TCEs on B cell depletion and reconstitution kinetics in AIDs. Furthermore, strong effects on the T cell compartment are observed, raising the intriguing possibility that their mechanism of action is not limited to the B cell depletion alone.
REFERENCES: [1] Bucci, L. et al. Nat. med. vol. 30,6 (2024): 1593-1601. doi:10.1038/s41591-024-02964-1.
[2] Hagen, M. et al. N Engl J Med 2024;391:867-869. DOI: 10.1056/NEJMc2408786.
Frequences of A. Naive, B. Memory, C. switched memory, and D. non switched memory B cells at short term follow-up (FU) and at 1-year follow-up (long term FU).
Expression of PD-1 in T cell subpopulations at baseline (t0), after the 3rd injection (t1), at 14-days (t2) and 1-month follow up (t3) and at 3-months follow up (FU3M)
Acknowledgements: NIL.
Disclosure of Interests: Laura Bucci: None declared, Tobias Rothe: None declared, Kirill Anoshkin: None declared, Danae-Mona Nöthling: None declared, Ann-Kathrin Götz: None declared, Markus Eckstein: None declared, Maria Gabriella Raimondo: None declared, Georg Schett BMS, Cabaletta, Johnson & Johnson, Kyverna, Novartis, UCB, Ricardo Grieshaber-Bouyer Bristol-Myers Squibb, Cullinan, Janssen, Lilly and Novartis, Astra Zeneca and Kyverna.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (