

Background: Anti-dsDNA antibodies play a crucial role in the pathogenesis of lupus nephritis. We have shown that dsDNA-specific staining of plasma cells can be performed using a double-stranded polynucleotide with a length of 100 base pairs (dsPN). We have shown in a proof-of-concept study that antigen-specific plasma cell staining is possible [1]. the approach was adapted using dsPN to deplete dsDNA-specific plasma cells in NZB/W mice.
Objectives: To investigate the selective depletion of dsDNA-specific plasma cells in NZB/W mice, a mouse model for lupus nephritis.
Methods: A conjugate of dsPN and an anti-mouse CD138 antibody with a human IgG1 heavy chain constant region (clone: REA104; Miltenyi Biotec) was prepared using a biotin-streptavidin interaction. The in vivo labelling of this conjugate on plasma cells was investigated 0, 2, 24, 48, 72 and 96 hours after its intraperitoneal (i.p.) administration in 16-week-old NZB/W mice (200 µg/mouse) by flow cytometry. The efficacy of plasma cell depletion in spleen, bone marrow and kidney was investigated two days after a single i.p. injection of the conjugate (200µg/mouse) to 16-18 week old NZB/W mice. Long-term treatment was initiated in NZB/W mice at 20 weeks of age by i.p. administration of the conjugate and continued every fortnight. These mice were immunised with ovalbumin 10 weeks before the start of treatment in order to demonstrate the selectivity of depletion. Proteinuria was monitored as a biomarker of nephritis. Antibody levels were monitored by ELISA.
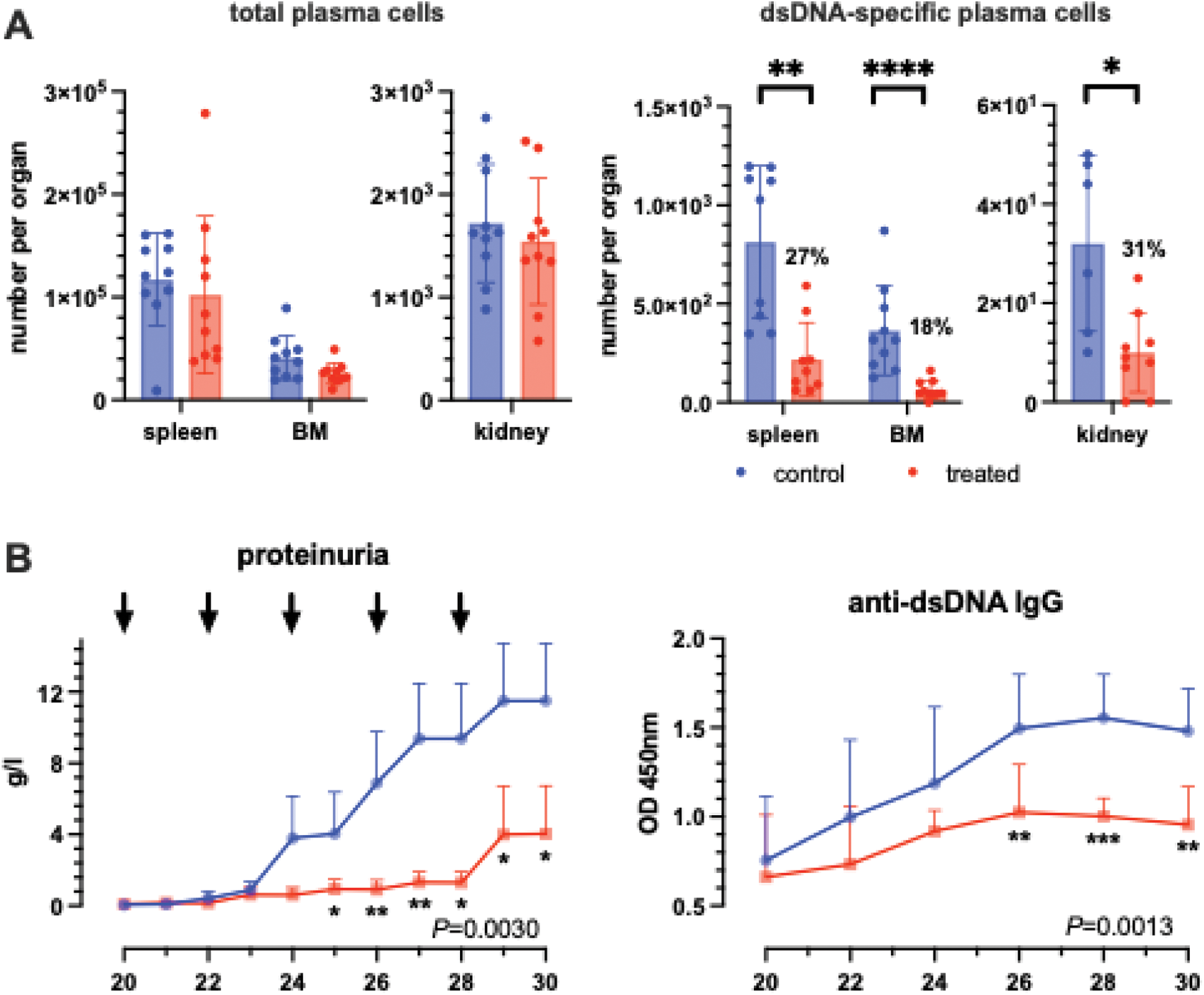
Results: Flow cytometric analysis showed that the conjugate selectively labelled plasma cells in both the spleen and bone marrow, while B or T cells were only minimally labelled or not labelled at all. The labelling peaked after 2 hours and then gradually decreased, with the signals disappearing completely from the surface of the plasma cells after 48 hours. The single administration of the conjugate led to a significant decrease in dsDNA-specific plasma cells by up to 80 % in the spleen, bone marrow and kidneys, while the number of total plasma cells and ovalbumin-specific plasma cells remained unchanged. As early as two weeks after the start of long-term treatment, anti-dsDNA antibodies began to drift apart between the treated and untreated groups, with significant differences in weeks 26-30, while anti-ovalbumin antibodies remained unchanged in both groups. At the start of treatment, proteinuria was low in both groups (average 0.05 g/l). While proteinuria in the treated group increased only slightly to an average of 1.3 g/l by week 28, there was a steady increase in the untreated group (average 9.4 g/l at week 28). Due to the development of drug antibodies against the human Fc1 portion of the anti-CD138 antibody and streptavidin, the follow-up was interrupted at week 30 (Figure 1).
Conclusion: If the selective depletion of dsDNA-specific plasma cells can be optimised and translated to SLE patients, this would be an important step towards personalised, precise treatment while preserving humoral immunity. The results support the application of this therapeutic concept to other (auto)antibody-mediated diseases.
REFERENCES: [1] Cheng, Q et al.: Eur. J. Immunol. 50, 284-291 (2020).
Therapeutic effect of the i.p. administration of the anti-CD138/dsPN conjugate in NZB/W mice. A ) In vivo depletion of anti-dsDNA-specific plasma cells in the spleen, bone marrow and kidney from NZB/W mice (n=10 per group) 48 hours after the i.p. administration of the conjugate. B) Effect of repeated treatments (arrow) of NZB/W mice (control group n=8, treated group n=7) on the proteinuria and levels of anti-dsDNA-antibodies compared to the untreated group.
Acknowledgements: Supported by Leibniz transfer (T85/2020) and Deutsche Forschungsgemeinschaft (TRR130).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (