

Background: Patients with granulomatosis with polyangiitis that are nasal carriers for Staphylococcus aureus are at higher risk of disease relapse [1]. The molecular basis of this association is yet unknown. Recent studies have investigated how microbial antigens might contribute to the activation of autoreactive B cells in such conditions. Previously, molecular mimicry has been shown for autoantibodies to lysosomal membrane protein-2 (LAMP-2), inducing pauci-immune focal necrotizing glomerulonephritis in rats, and bacterial adhesin FimH [2]. Whether antibodies directed toward S. aureus antigens could react against the autoantigen proteinase 3 (PR3) in patients with ANCA-associated vasculitis (AAV), thus explaining the link between S. aureus nasal carriers and relapses, has not been investigated, yet.
Objectives: This study is aimed at determining whether anti- S. aureus antibodies can cross-react with the human autoantigen PR3, potentially disrupting immune tolerance and triggering autoimmune flares in susceptible individuals.
Methods: Indirect enzyme-linked immunosorbent assay (ELISA) was used for detection of IgGs directed against PR3 antigen, PR3-unrelated antigens, and specific S. aureus antigens in sera obtained from either PR3-ANCA positive AAV patients (PT, n=6), healthy donors (HD, n=3), or mice challenged with S. aureus . Challenged mice were used to test S. aureus antigen specific antibodies in an unbiased way as mouse PR3 is non-homologous to human PR3 and it is not immunogenic in mice, therefore mouse anti-PR3 antibodies do not develop naturally. Sera were collected from two groups of mice (n=8 each) at 5 (group 1) and 15 (group 2) days post-infection with S. aureus . Animals were infected intravenously into the retroorbital nexus with 5x10 6 CFU S. aureus USA300. Infected animals carried an average of 10 7 -10 8 CFU/g of kidney tissues on day 15 post infection, when all animals were sacrificed. ELISA plates were coated with human recombinant PR3 (rPR3), PR3-unrelated proteins (S1 subunit of Spike protein of SARS-CoV-2, Streptolysin O (SLO) protein of Group A Streptococcus [3, 4]), α-hemolysin (Hla H35L ) and Staphylococcal protein A (SpA KKAA ) [5, 6]. Following serum incubation, the presence of IgG antibodies was detected using an enzyme-conjugated secondary antibody specific to human or mouse IgGs, respectively. Data are presented as the net Optical Density values at 405 nm (Net OD 405nm ) measured after 30 minutes of colorimetric substrate reaction.
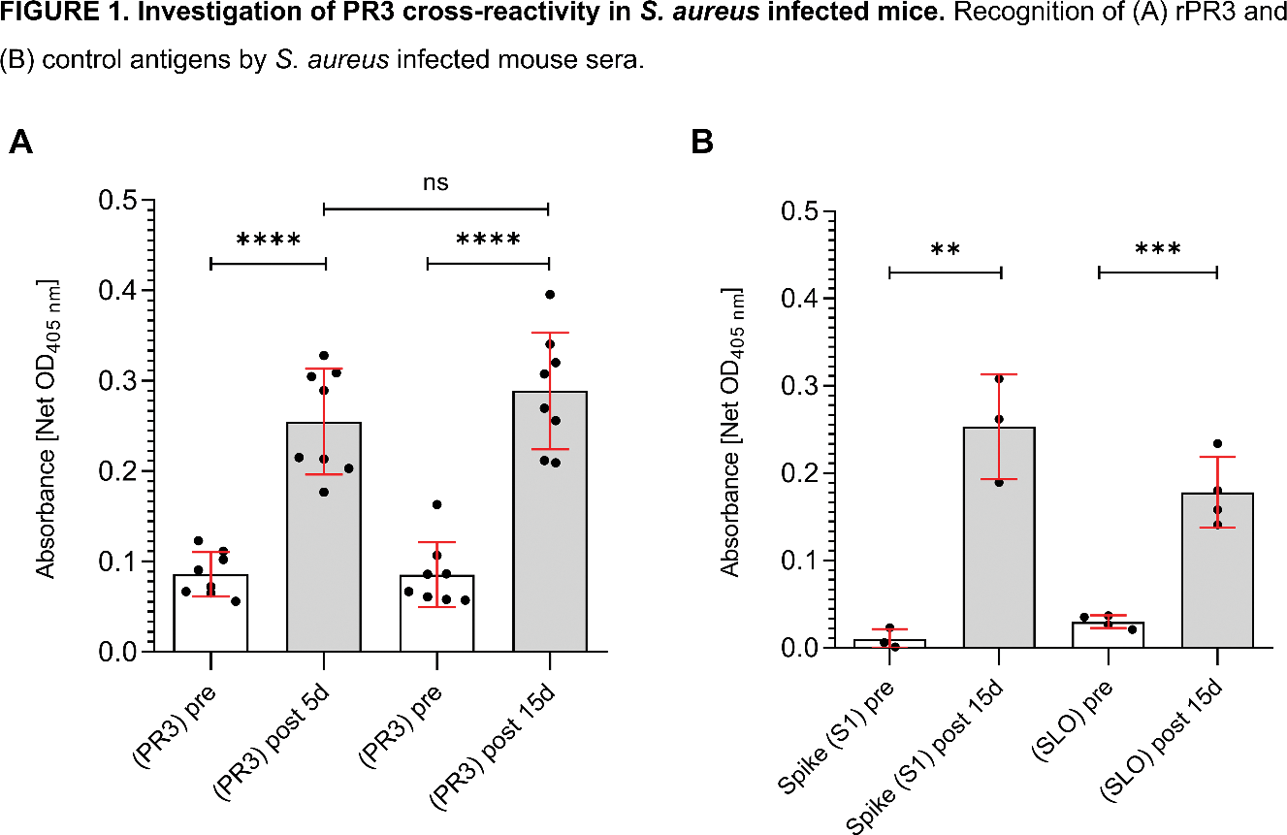
Results: PT sera showed significant reactivity to rPR3 (3.607 (IQR: 2.286–4.000)), whilst no reactivity was observed in healthy donor sera (0.4456 (IQR: 0.3863–0.6037)), as expected (p<0.0238). No difference was observed between PT and HD when tested for the S. aureus FhuD2 antigen (1.712 (IQR: 1.151–2.521) vs 2.481 (IQR: 0.8202–4.000), p=0.5476). To overcome this confounding effect of immune response following S. aureus exposure in both PTs and HDs, precluding any recognition of possible cross-reactivity between PR3 and S. aureus antigens, mice challenged with S. aureus were used. IgG recognizing human rPR3 at 5- and 15-days post infection were detectable in mice sera and were significantly higher as compared to sera of naïve animals (2-day prior to infection) (0.086±0.004, 0.086±0.009 p<0.0001, Figure 1A) with no difference in IgG titers between the 5- (0.25±0.024) and 15-days (0.29±0.003) post-infection groups (p>0.05). Seroconversion to S. aureus antigens Hla H35L and SpA KKAA , was also observed in sera collected on days 5 and 15 post infection with ELISA (p<0.0001 when compared to naïve sera). To assess whether the observed cross-reactivity with PR3 was specific to rPR3, the reactivity toward SLO and Spike S1 was also compared between sera from naïve and infected animals (n=3 for Spike S1, n=4 for SLO). The reactivity against both these two control antigens at 15 days post infection was similar (p<0.0001 for both comparisons, yet non-significantly lower to the reactivity observed against human rPR3 (Figure 1B).
Conclusion: This study provides insights into the potential antibody cross-reactivity between anti- S. aureus and anti-PR3 antibodies in the context of AAV. Naïve mice and animals challenged with S. aureus were used to eliminate potential confounding factors with PT and HD sera, showing that anti-PR3 IgG levels were detectable at low titers in sera of challenged animals. Further analyses revealed similar reactivity pattern against unrelated antigens, indicating that the signal observed for human rPR3 may be non-specific. These results suggest that exposure to S. aureus in mice triggers broad non-specific immune responses that may not be related to molecular mimicry between S. aureus and rPR3. Funding: This study was supported by a research grant from the Vasculitis Foundation to Dr. Alvise Berti and CureAAV Initiative sponsored by NorthStar Medical Radioisotpes.
REFERENCES: [1] Salmela, A., et al. Rheumatology, 2017.
[2] Kain R, et al. Nat Med. 2008.
[3] Huang, et al. Acta Pharmacol Sin,2020.
[4] Chiarot E, et al. mBio 2013.
[5] Bhakdi, S et al. Microbiol. Rev. 1991.
[6] Falugi, F. et al. mBio 2013.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (