

Background: ANCA-associated vasculitis (AAV) is a severe autoimmune disease targeting small vessels with significant kidney involvement (AAGN – ANCA-associated glomerulonephritis). Despite current therapies, up to 28% of patients progress to end-stage renal disease, and many die due to infectious complications. More targeted therapies have the potential to improve outcomes and decrease the rate of infectious complications.
Objectives: To facilitate the development of novel therapeutic targets, we aimed to identify the major disease-driving molecular pathways in AAGN. To achieve this aim, we performed transcriptomic analysis of kidney biopsies from 22 patients with AAGN and 5 healthy controls.
Methods: RNA sequencing was performed on RNA extracted from clinically indicated FFPE kidney biopsies. Five pre-implantation donor kidney biopsies were used as controls. Identification of differentially expressed mRNA and pathway analysis was performed using DESeq2 and gene set enrichment analysis (GSEA), respectively. Deconvolution of gene expression data to identify involved immune cell populations was performed using Cibersort. Gene sets correlation with histological changes in the kidney were used for pathway analysis.
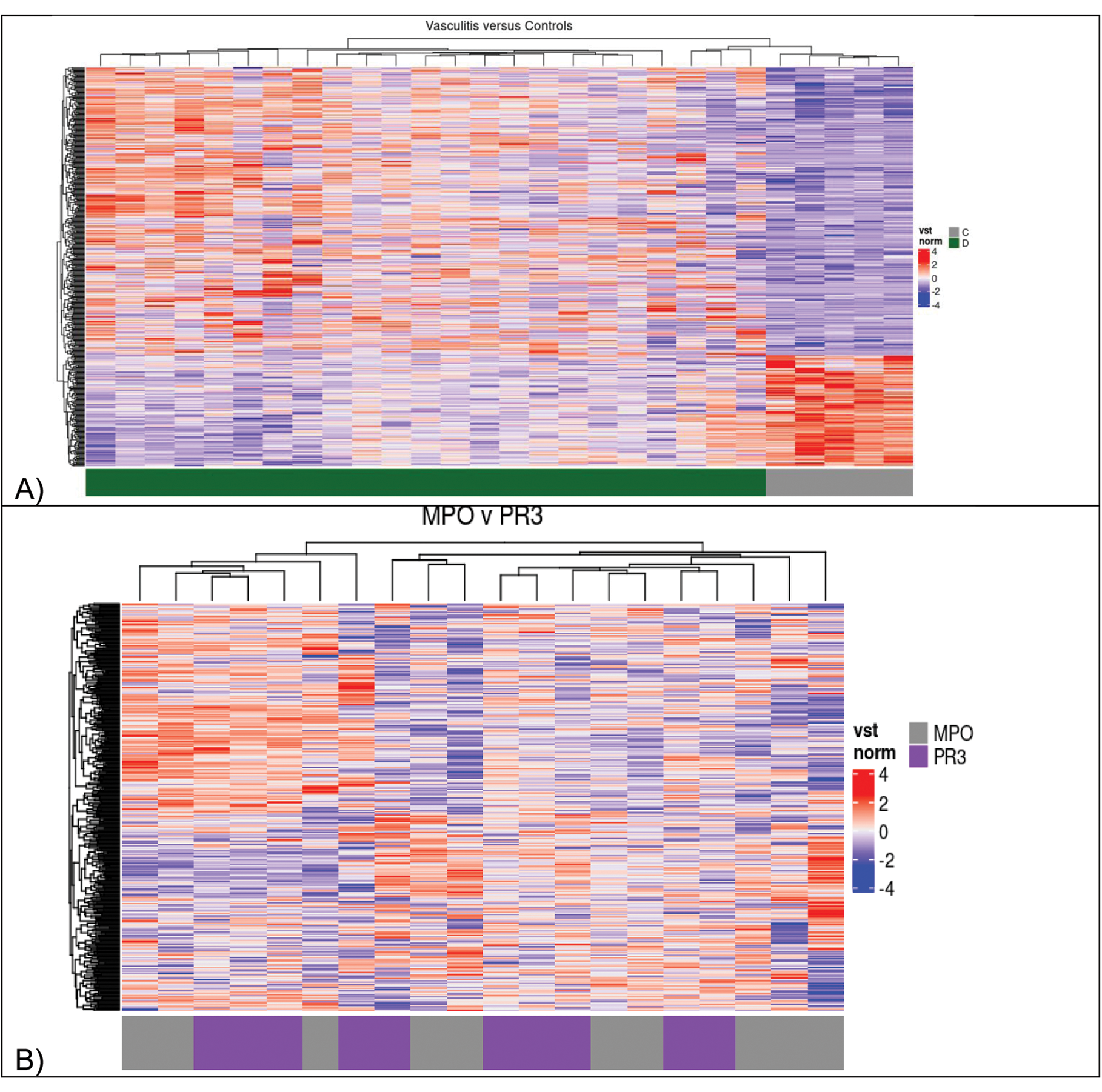
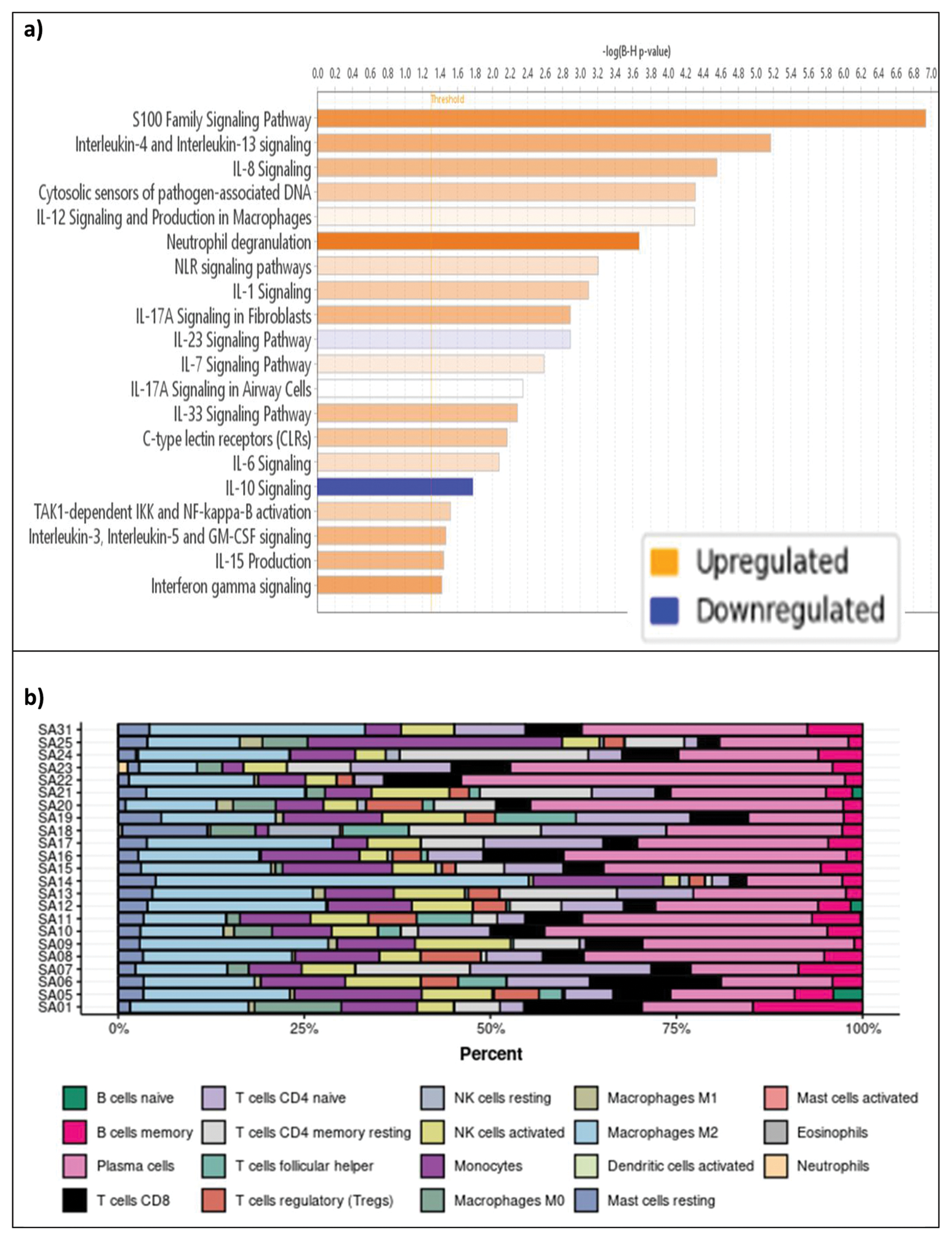
Results: Our cohort was predominantly female (13/23), White (22/23), and had a median age of 58 years. Compared to controls, 491 genes were upregulated and 297 downregulated in AAV patients (log2 fold change > 1.0, FDR < 0.05). Anti-MPO+ patients displayed 196 upregulated genes and 135 downregulated genes, while anti-PR3+ patients had 413 upregulated and 339 downregulated genes compared to controls. AAV samples segregated from controls but anti-MPO versus anti-PR3 positive subgroups did not (Figure 1). Compared to controls, patients with AAGN demonstrated upregulation of several immune markers such as IL-17D, IL-8RBP, IL-7R, IL-24, CD163, CD19, CD244, CD48, and CD6; and downregulation of IL-10RA. GSEA also revealed upregulation of several immune pathways including IL-18, IL-12 signaling and production in macrophages, IL-17A signaling in fibroblasts, IL-17A signaling in airway cells, and downregulation of IL-10 signaling (Figure 2a). Enrichment of genes associated with pattern recognition, antigen binding, and MHC class protein binding was consistently seen. Deconvolution by Cibersort confirmed the presence of diverse immune cell populations, including NK cells, M2 macrophages, and plasma cells in kidney tissue of patients with AAGN (Figure 2b). Lastly, we identified several immune pathways that correlate with specific renal histological changes. Complement cascade and SUMOylation of chromatin organization proteins were found to correlate to the percentage of normal glomeruli. STAT3, TRIM25, and IKZF1 pathways correlated to the degree of glomerular sclerosis. IL-23 signaling and production in macrophages, IL-12 signaling and production in macrophages, IL10, IL2, and IL18 pathways correlated to the number of glomeruli with necrotizing changes. Lastly, FAT10 signaling and class I MHC medicated antigen processing and presentation correlated to the degree of interstitial fibrosis and tubular atrophy.
Conclusion: Our data demonstrates that anti-MPO and anti-PR3 positivity does not appear to segregate patients on a transcriptomic renal tissue level. Our data provide evidence of several immune cell populations existing within the kidney with AAN. Our data identified several possible therapeutic targets that warrant further study in AAN including but not limited to anti-JAK, TNF, IL-17, and IL-8 agents. Correlation to renal histology identified several unique immune mediated inflammatory mechanisms for each histologic subtype spanning innate and adaptive immunity. Further investigations may facilitate improved stratification of patients and guide therapeutic development.
REFERENCES: [1] Booth, A. D. et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. American journal of kidney diseases 41, 776-784 (2003).
[2] Stone, J. H. et al. Etanercept combined with conventional treatment in Wegener’s granulomatosis: a six-month open-label trial to evaluate safety. Arthritis Rheum 44, 1149-1154, doi:10.1002/1529-0131(200105)44:5<1149::Aid-anr197>3.0.Co.
[3] Gan, P.-Y. et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. Journal of the American Society of Nephrology 21, 925-931 (2010).
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (