

Background: Age is the most significant risk factor for giant cell arteritis (GCA), primarily affecting individuals over 50, with peak onset between 70 and 80 [1]. Aging-related changes, such as vascular remodeling and immunosenescence, are believed to drive this age-dependent pattern [2], however, the precise mechanisms remain poorly understood. Emerging evidence suggests that biological age, reflected by markers like leukocyte telomere length (LTL) attrition and epigenetic age acceleration (EAA), may better explain susceptibility to GCA. These markers, which provide a more precise measure of an individual’s aging rate, serve as robust predictors of health outcomes and age-related disease risk [3]. Given the pivotal role of aging in vascular remodeling and immune system dysregulation in GCA, genetic factors influencing biological aging markers, including telomere shortening and EAA, might contribute to GCA susceptibility.
Objectives: This study aimed to explore the shared genetic architecture between GCA and markers of biological aging, specifically LTL and EAA. By conducting a cross-trait meta-analysis of genomic data, we sought to identify genetic factors that influence both biological aging and GCA risk, thereby providing new insights into the role of aging in the pathogenesis of this vasculitis.
Methods: Summary statistics comprising approximately 6.6 million genetic variants were retrieved from previously published GWASs conducted for GCA (3,498 cases and 15,550 controls), LTL (472,174 individuals), and EAA (34,710 individuals). A cross-trait meta-analysis was conducted using the Association analysis based on SubSETs (ASSET) R package to identify variants with pleiotropic associations between GCA and markers of biological aging. Specifically, ASSET systematically evaluates the associations of genetic variants across all possible subsets of traits. This method identifies the subset that contributes most significantly to the overall association signal and quantifies its statistical significance. Variants reaching genome-wide significance (p<5×10⁻⁸) were then subjected to functional annotation and prioritization of likely causal genes using FUMA, providing insights into their potential biological relevance. Finally, the DrugBank database was queried to identify candidate drugs targeting the prioritized genes, which could potentially be repurposed for GCA treatment.
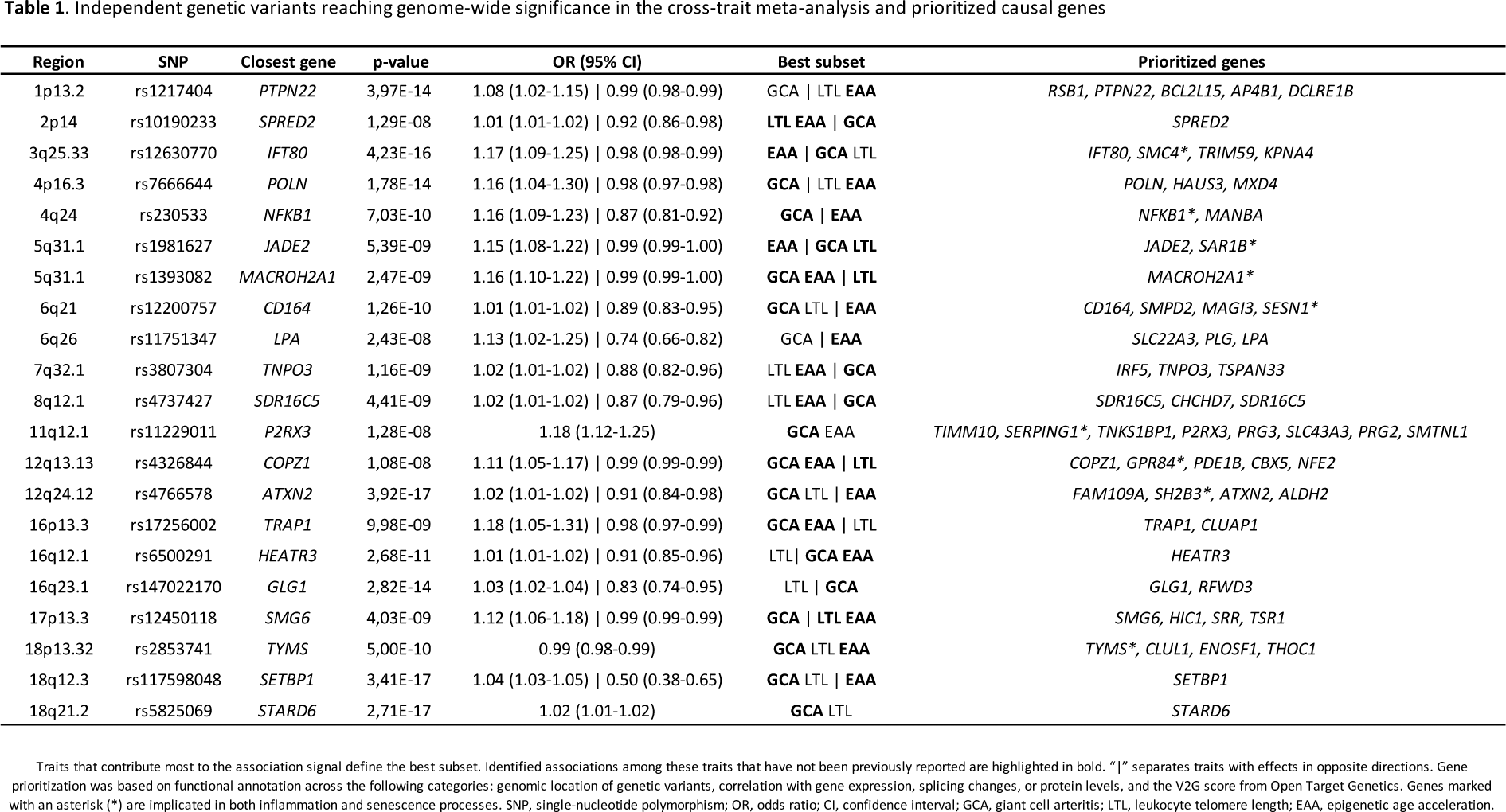
Results: This cross-trait meta-analysis identified 21 independent genetic variants shared between GCA and at least one aging marker, with 16 of them shared across all traits (Table 1). Functional annotation revealed that, although located in non-coding regions, these variants influence gene expression in tissues relevant to GCA, enabling the prioritization of potential causal genes (Table 1). Two pleiotropic signals were identified at
PTPN22
and
PLG
, both known risk factors for GCA, while the remaining variants represent potentially novel susceptibility loci for this vasculitis. Interestingly, several prioritized genes, including
SERPING1
,
SAR1B
,
SESN1
,
SH2B3
, and
SMC4
, among others, are implicated in both inflammation and senescence, providing new insights into the molecular pathways linking aging and GCA. Notably, 43% of the shared variants exhibited opposing effects on GCA and aging markers, suggesting that the same genetic variants may play distinct roles depending on the biological context. Finally, drug repurposing analysis identified promising therapeutic candidates for GCA, such as pazopanib, an angiogenesis inhibitor, and investigational drugs targeting the NF-κB pathway, expanding the therapeutic potential for GCA treatment.
Conclusion: This study revealed a significant genetic overlap between GCA and biological aging markers, uncovering shared molecular pathways that link inflammation and aging. These findings pave the way for the development of novel therapies targeting both inflammation and cellular senescence in GCA.
REFERENCES: [1] Watts RA, Hatemi G, Burns JC, Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18(1):22–34.
[2] Gloor AD, Berry GJ, Goronzy JJ, Weyand CM. Age as a risk factor in vasculitis. Semin Immunopathol. 2022;44(3):281–301.
[3] Moqri M, Herzog C, Poganik JR, Justice J, Belsky DW, Higgins-Chen A, et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023;186(18):3758–75.
Acknowledgements: We thank Sofía Vargas and Gema Robledo for their excellent technical support, and the participants for their collaboration. This work was supported by grant PID2022-136416OB-I00 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”, and Redes de Investigación Cooperativa Orientadas a Resultados en Salud (RD21/0002/0039 and RD24/0007/0016) funded by Instituto de Salud Carlos III (MCIN). L.O.F is recipient of a Ramon y Cajal fellowship (RYC2022-036635-I) funded by MICIU/AEI/10.13039/501100011033 and by ESF+. I.R.M was supported by the program FPU (FPU21/02746), funded by the Spanish Ministry of Universities. G.B.Y.’s contract is part of the grant PREP2022-000712, funded by the MCIN/AEI/10.13039/501100011033 and the ESF+. This research is part of the doctoral degree awarded to L.M.G., within the Biomedicine program from the University of Granada. This work was also supported in part by the Medical Research Council (MR/N011775/1), the NIHR Leeds Biomedical Research Centre (NIHR203331) and NIHR Senior Investigator award to AWM (NIHR202395). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (