

Background: PD-1 is an inhibitory receptor expressed on activated T cells and at high levels on T follicular and peripheral helper (Tfh/Tph) cells. Tfh/Tph are enriched in autoimmune diseases and support B cell function. JNJ-67484703 is an IgG1 monoclonal antibody (mAb) designed to agonise PD-1 and deplete cells with high PD-1 levels. Experience shows that peripheral cellular depletion with mAbs is not always accompanied by effective tissue depletion.
Objectives: To investigate the biological effects of multiple doses of JNJ-67484703 in disease-relevant tissues in participants with rheumatoid arthritis (RA), ulcerative colitis (UC) and Sjogren’s disease (SjD).
Methods: This phase 2 open label proof of biology trial aimed to recruit 15 patients in each of 3 disease groups (RA, UC and SjD) to receive 7 doses of subcutaneous JNJ-67484703 at weeks 0, 1, 2 and then every 2 weeks until week 10. Within each disease, participants were allocated to one of two doses with predicted non-overlapping exposures: 3 mg/kg (n=10) or 0.5 mg/kg (n=5). Key inclusion criteria for RA were swollen joint ≥4, CRP ≥3 mg/l and an inadequate response to conventional DMARD therapy ± biological therapy. Key criteria for UC were moderately to severely active disease (Mayo score 6-12 and ≥2 on endoscopy subscore) and failure of at least one advanced therapy. Key criteria for SjD were anti-Ro/SSA positive, activity in ESSDAI biological domain or low lymphocyte count due to SjD, ESSPRI ≥5 and stimulated salivary flow >0 mls/min. Synovium, gut or minor salivary gland biopsies were taken at weeks 0 and 12 (final efficacy visit). The primary outcome was change in the proportion of PD-1 hi CD4+ T cells (Tfh/Tph) in disease relevant target tissue, measured by scRNAseq with feature barcoding and analysed with Bayesian hierarchical modelling. Safety was assessed until Week 24. Adverse event reporting included any blood laboratory result outside the normal range, regardless of clinical significance.
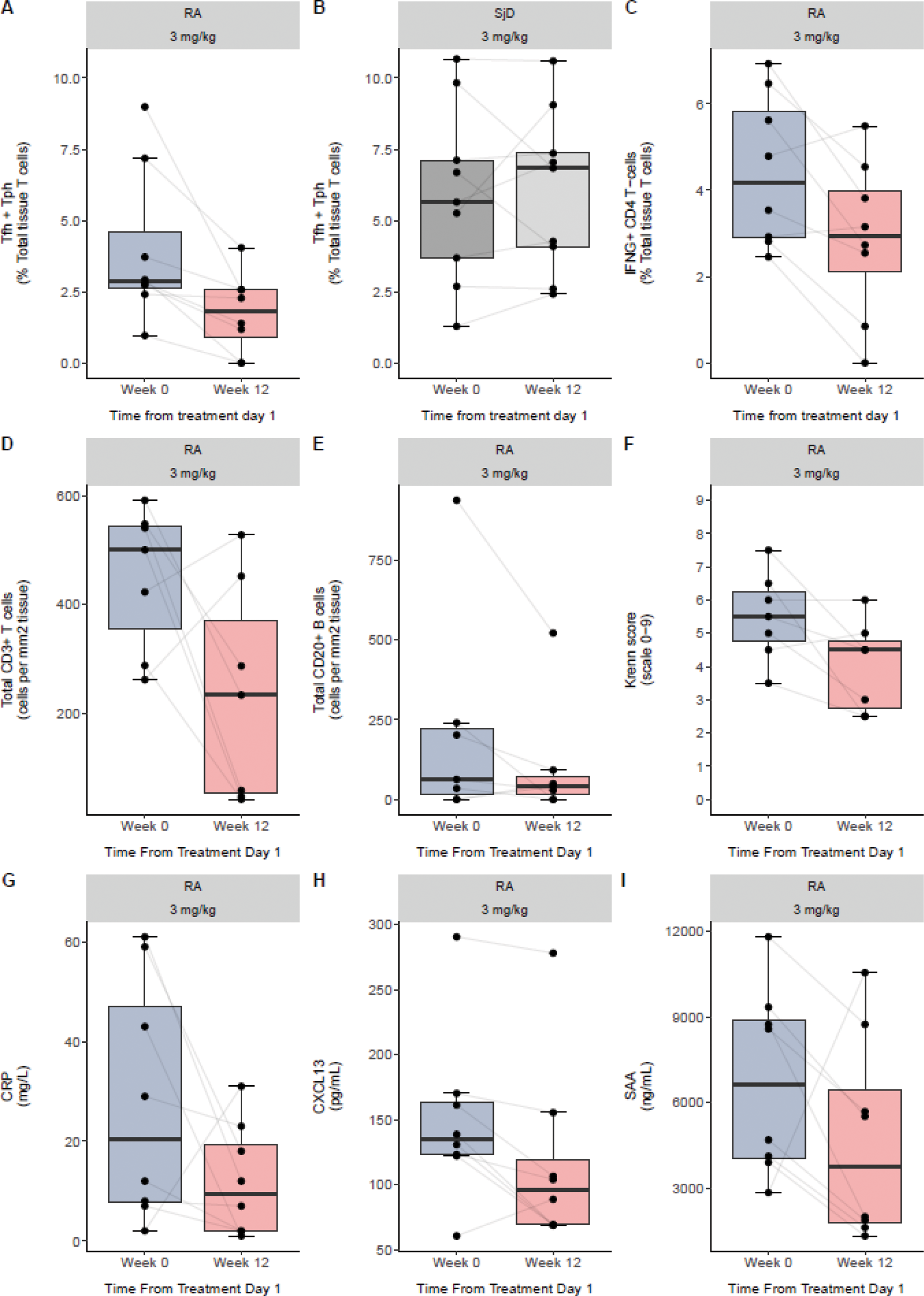
Results: A total of 37 patients were recruited (RA n=17, UC n=5 and SjD n=15). Two RA participants withdrew by choice prior to second biopsy and were replaced. Three UC patients withdrew from active treatment due to unsatisfactory response at 5, 6 and 7 weeks after receiving 3 (n=1) or 5 (n=2) doses, but provided end of treatment biopsies. Due to slow recruitment and high withdrawal rate, the UC arm was terminated early. Four patients were not included in the primary outcome analysis due to failure of QC in at least one sample [RA 3 mg/kg (n=2), UC 3 mg/kg (n=1), and SjD 3 mg/kg (n=1)]. Baseline median (IQR) Tfh/Tph as a proportion of total tissue CD3 T cells were 2.8% (1.0, 3.7) in RA, 5.4% (3.8, 7.0) in SjD and 14.9% (13.0, 17.0) in UC. Combining all disease cohorts, there was a 28% and 43% posterior probability of a decrease in Tfh/Tph over time in disease tissues at the lower and higher doses, respectively; however pre-planned sensitivity analyses showed large variation dependant on disease (Table 1). The greatest effect was observed in the 3 mg/kg RA cohort, where there was a 96% posterior probability of Tfh/Tph decrease over time (median change in percentage, -0.14 per week; Credible Interval, -0.27, -0.01). Tfh/Tph decrease was accompanied by median reductions in the following: proportion of scRNAseq IFNγ+CD4+ T cells, total T and B cell counts per mm 2 as assessed by immunofluorescence, Krenn synovitis score [5.5 (IQR 4.8-6.3) to 4.5 (IQR 2.8-4.8)], and CRP, CXCL13 and serum amyloid A (SAA) (Figure 1). A trend for median reduction in DAS-28(CRP) score from baseline was observed at Week 12: -0.6 (IQR -2.1, 0.0) with 0.5 mg/kg and -1.8 (IQR -2.1, 0.3) with 3 mg/kg treatment. Change in tissue Tfh/Tph correlated with change in DAS-28(CRP). For SjD, no decrease over time in Tfh/Tph cells was observed. Although improvement in ESSPRI symptom scores were observed in both dose groups, there were no histopathological improvements and only 33% STAR composite index responders with 3 mg/kg. In peripheral blood, median (IQR) CD3+PD1 hi T cell count in the 3 mg/kg groups was greater in SjD than RA at baseline [68/μL (45, 83) vs 25/μL (17, 29)] and week 12 [25/μL (23, 35) vs 3/μL (2, 10)] despite similar change from baseline [-31 (-51, 2) vs -18 (-24, -13)]. Similar trends were observed for PD1 hi Tfh/Tph cell counts. Pharmacokinetics of JNJ-67484703 were similar between RA and SjD. The majority of adverse events were grade 1 (mild; 91.7%) or grade 2 (moderate; 8.0%). There was one grade 3 event of worsening colitis in UC and one asymptomatic grade 4 exercise-related creatine kinase elevation. There were no serious adverse events or deaths.
Conclusion: In RA, but not SjD or UC, 3 mg/kg JNJ-67484703 was associated with a reduction in PD1 hi Tfh/Tph in target tissue and other parameters of tissue inflammation, demonstrating the value of early proof of biology studies. Overall, JNJ-67484703 appeared safe and well-tolerated.
REFERENCES: NIL.
Posterior distribution values of the change in PD-1 hi Tfh/Tph value per week
| Effect of time: 0.5 mg/kg | Effect of time: 3.0 mg/kg | |||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Mean | Median | CrI (95%) | P(<0) | Mean | Median | CrI (95%) | P(<0) |
| Main Model | ||||||||
| Pooled | 0.08 | 0.08 | -0.13, 0.30 | 28% | 0.02 | 0.02 | -0.14, 0.16 | 43% |
| Sensitivity Models | ||||||||
| RA | -0.11 | -0.11 | -0.28, 0.05 | 88% | -0.14 | -0.14 | -0.27,
| 96% |
| UC | NA | NA | NA, NA | NA | 0.53 | 0.53 | -0.04, 1.09 | 6% |
| SjD | 0.15 | 0.15 | -0.10, 0.38 | 15% | 0.02 | 0.02 | -0.17, 0.20 | 43% |
Crl, Credible Interval; P(<0), posterior probability of a decrease in percentage of Tfh/Tph over time
Primary outcome in RA and SjD 3 mg/kg arms (A,B), and in the RA 3 mg/kg arm: IFNγ+CD4+ T cells as proportion of total tissue T cells (C), total tissue T and B cells as measured by immunofluorescence (D,E). Krenn histopathological synovitis score (F), serum CRP, CXCL13 and serum amyloid A (SAA) (G-I).
Acknowledgements: NIL.
Disclosure of Interests: Benjamin A. Fisher Novartis, Servier, Novartis, BMS, Servier, Galapagos, Roche, UCB, Sanofi, Johnson & Johnson, AstraZeneca, Otsuka, Amgen, Kiniksa, Johnson & Johnson, Servier, Galapagos, Celgene, Novartis, Joseph van de Wiel: None declared, Jason D. Turner: None declared, Yuxin Susan Liu GSK, Johnson & Johnson, SynAct, Tom Hosack: None declared, Jennie Young: None declared, Saly Al-Taei: None declared, Aliaksandra Baranskaya: None declared, Frances Humby UCB, Roche, Pfizer and Genentech, Pfizer, Jack McMurray: None declared, Sebastian Gilbert AstraZeneca, Christopher Shave UCB, David H. Gardner: None declared, Saba Nayar: None declared, Toby Cox: None declared, Roja Muthinti: None declared, Alberto Recchioni: None declared, Mark C. Maybury: None declared, Paola de Pablo: None declared, Simon Bowman Abbvie, Aera, Amgen, Argenx, Aurinia, Bain, BMS, EcoR1, Galapagos, Iqvia, J&J, Kiniksa, Novartis, Scitaris, Anna Rowe: None declared, Christopher D Buckley GSK, Mestag Therapeutics, GSK, Roche, Johnson & Johnson, AbbVie, Takeda, Pfizer, Eli-Lilly, Roche, Johnson & Johnson, GSK, Takeda, Pfizer, Celsius, Alex Richter CSL, Takeda, MSD, AstraZeneca, GSK, Matthew J Loza Johnson & Johnson, Johnson & Johnson, Stanley Marciniak Johnson & Johnson, Johnson & Johnson, Wan-Fai Ng Novartis, Johnson & Johnson, Bristol Myers Squibb, Sanofi, Argenx, IQVIA, Quotients, Resolve Therapeutics, Veloxis and EQT, Arthur Pratt GSK, Simon Travis BMS, Ferring, Janssen, Lilly, Pfizer, Sun Pharma, Takeda, Alimentiv; Apexian; Apollo; Arcturis; AstraZeneca; BMS; Clario; Cosmo; Endpoint Health; EQrX; Equillium; Ferring; Galapagos; Genentech/Roche; Gilead; GSK; Johnson & Johnson; Lilly; Mestag; Microbiotica; ONO; Pfizer; Phesi; Protagonist; Sanofi; Satisfai; Sensyne Health; Takeda; Theravance; Tr1X Bio, AbbVie, Celgene, Celsius, ECCO, Galapagos, IOIBD, Janssen, Lilly, Pfizer, Takeda, UKIERI, Vifor, Victoria Homer Acticor, Andrew Filer Johnson & Johnson and Sonoma, BMS, Roche, UCB, Nascient, Mestag, GSK, Johnson & Johnson and Synact.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (