

Background: Since 2016, the mySCQM app has been available for patients with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), and psoriatic arthritis (PsA) enrolled in the Swiss Clinical Quality Management (SCQM) registry to monitor their disease closely. By recording and graphically displaying monthly patient-reported outcomes (PROs) alongside medication use, the app offers a practical and valuable tool for self-observation between clinical consultations [1, 2].
Objectives: This study aimed to (1) describe the population of mySCQM app users, (2) quantify app retention among RA, axSpA, and PsA patients and distinguish between regular vs. occasional users, and (3) identify baseline predictors of app discontinuation.
Methods: In this retrospective study of prospectively collected data from the SCQM registry, baseline was defined as the date of the first recorded entry in the mySCQM app. The following patient characteristics were collected: sex, age at first symptoms, age at diagnosis, age at first app use, as well as the following variables at baseline: disease duration, highest achieved educational level (primary, secondary, tertiary), body mass index (BMI), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Patient Global Assessment (PtGA; 0–10), and Pain Visual Analog Scale (VAS; 0–10). To quantify app retention, we used the Kaplan-Meier estimator to determine the probability of continuous app use. Discontinuation was defined as a six-month gap with no mySCQM entries. We then applied univariate Cox proportional hazards models within each disease (RA, axSpA, PsA) to evaluate potential baseline predictors of app discontinuation. Predictors included sex, age at first app use, educational level (primary, secondary, tertiary), disease duration, and PtGA at baseline. These analyses were restricted to patients with complete data in all predictors. Additionally, we assessed whether initial app use frequency was a predictor of app discontinuation. To this end, two frequency-of-use groups were defined: occasional and regular users, with 1–3 and 4 or more app entries, respectively, within the first six months following the initial app entry.
Results: We included a total of 3,950 patients of whom 38% had RA, 41% axSpA, and 21% PsA (Table 1). At the time of first app use, 61% of participants were female (76% in RA, 52% in axSpA, and 51% in PsA), with a median age of 50.3 years overall (RA: 56.1 years, axSpA: 43.9 years, PsA: 52.1 years). Of the participants, 11% reported primary education, 53% had secondary education, and 36% had attained a tertiary-level degree. Baseline characteristics aligned with the expected profiles for their respective diseases. Kaplan-Meier estimates revealed a median app retention of approximately 2.5 years across all patients, with 36% of users still entering data in the mySCQM app five years after their first entry. Among individual cohorts, PsA patients showed the highest median app retention at 3.1 years, followed by RA and axSpA patients at 2.6 and 2.1 years, respectively (Figure 1A). Cox proportional hazards analyses (Figure 1B) indicate that older age at first app use was associated with a lower risk of app discontinuation in axSpA and PsA, but not in RA. Higher educational levels (secondary or tertiary vs. primary) were consistently linked to longer app retention across the three diseases. Additionally, patients with higher PtGA at baseline tended to discontinue using the app sooner in RA and axSpA. In contrast, we did not detect evidence for an association of sex and disease duration on app retention, with the exception of RA patients, where longer disease duration was associated with an elevated risk of discontinuation. Patients who used the app regularly in the first six months had a four-fold lower risk of discontinuing self-observation thereafter: the median app retention from first entry was 5.6 years in regular users, whereas 50% of occasional users discontinued using the app within 8 months.
Table 1. Baseline characteristics at first app use. The missingness is given over all 3 diagnoses.
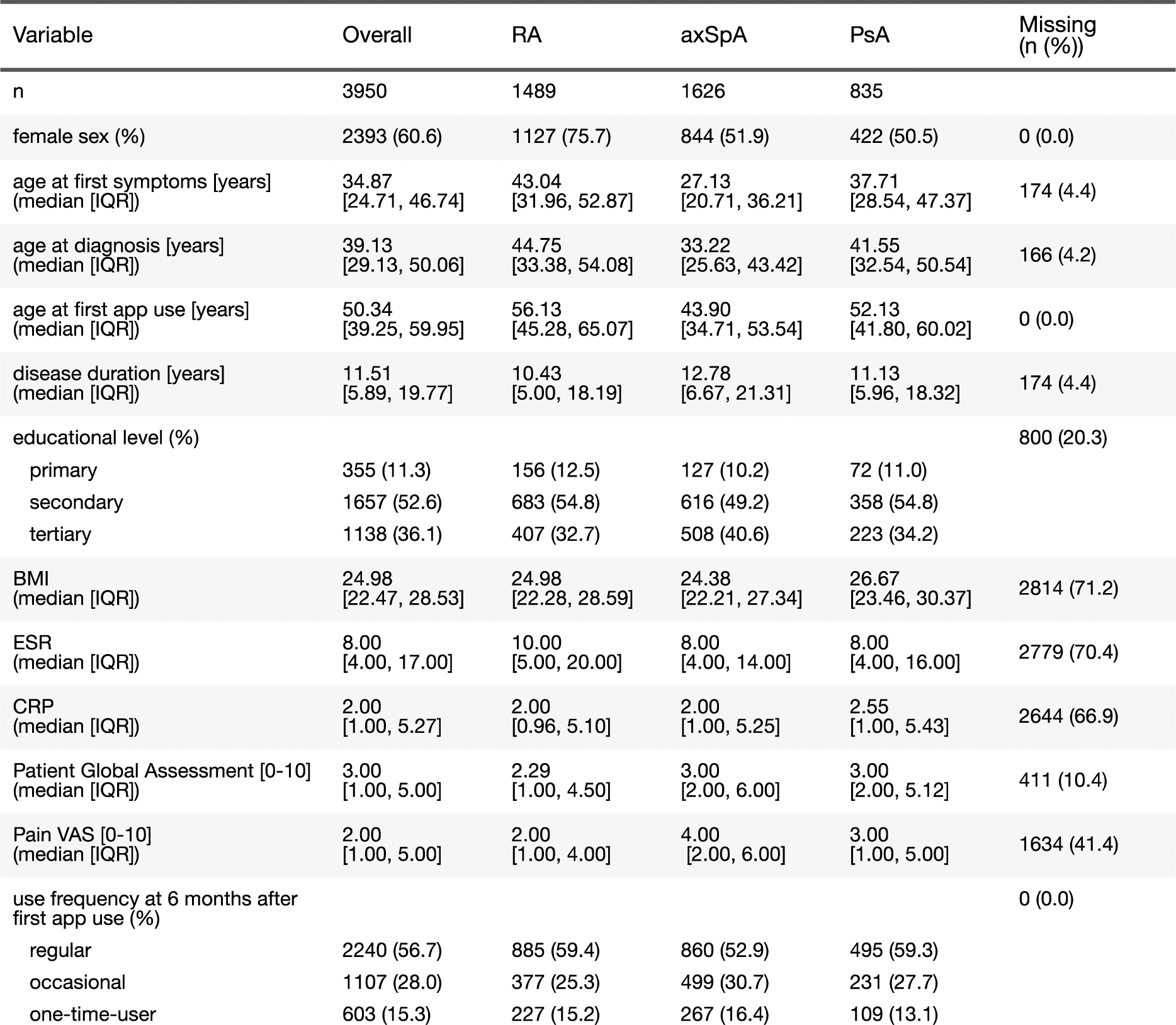
A ) Kaplan-Meier estimator with 95% confidence bands of the survival function for continuous app use for all 3 indications. B ) Univariate predictors at baseline for app retention by diagnosis: Shown are estimated hazard ratios (HR) and two-sided 95% confidence intervals. For age and disease duration, the HR refers to an increase roughly corresponding to the respective inter-quartile range (IQR). In case of PtGA, the HR for a 1-unit increase is presented, which is considered clinically relevant. Results denoted with the degree sign (°) were statistically significant at the 5% level. The analyses were based on the subset of patients with complete information on all predictors (RA: 1218, axSpA: 859, PsA: 629).
Conclusion: This first real-world study of long-term patient app retention in rheumatic diseases, analysing the mySCQM app within the SCQM registry, suggests a strong potential for digital health monitoring in patients who initially used the app regularly. Educational background and age were identified as key predictors of prolonged use. The study findings provide valuable insights for optimising digital health initiatives in inflammatory rheumatic diseases and potentially other chronic conditions. Moreover, they highlight the critical importance of early app engagement.
REFERENCES: [1] Shaw Y, et al. Impact of assessing patient-reported outcomes with mobile apps on patient–provider interaction. RMD Open 2021;7:e001566. doi: 10.1136/rmdopen-2021-001566.
[2] Raptis C E, et al The mySCQM patient app: insights from eight years of use for self-observation in the SCQM cohorts. Swiss Med Wkly. 2024;154:4175. doi: 10.57187/s.4175.
Acknowledgements: The SCQM thanks all patients participating in the registry. A list of rheumatology offices and hospitals that contribute to the SCQM registry can be found on
Disclosure of Interests: Jonas Brändli: None declared, Catherine Elizabeth Raptis: None declared, Myriam Riek: None declared, Almut Scherer: None declared, Anna Grabowski: None declared, Axel Finckh consultancies and speaker honoraria for AbbVie, AstraZeneca, Eli-Lilly, Gilead, MSD, Pfizer, Johnson & Johnson, Ines von Mühlenen: None declared, Burkhard Moeller: None declared, Andrea Rubbert-Roth: None declared, Thomas Hügle Patentholder for Detectra and board member of Atreon and Vtuls, Reserarch support: Fresenius Kabi, Eli Lilly and Pfizer. Honoraria, travel support or consulting fees from: Abbvie, GSK, Roche, Novartis, BMS, Eli Lilly, Janssen, UCB, Pfizer and Werfen, Raphael Micheroli: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (