

Background: Psoriatic arthritis (PsA) is an independent risk factor for developing cardiovascular (CV) disease. Results from a large meta-analysis indicated that CV disease risk is increased by 43% in patients with PsA compared with the general population [1]. The IL-23/T helper-17 pathway is recognized to play a fundamental role in PsA pathogenesis [2] and may be implicated in the development of cardiometabolic comorbidities [3]. Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor with a unique mechanism of action distinct from Janus kinase 1,2,3 inhibitors. Treatment with deucravacitinib has shown clinical efficacy in a randomized, double-blind, placebo-controlled, phase 2 study of patients with active PsA (NCT03881059).
Objectives: In this post hoc analysis, we investigated the impact of deucravacitinib treatment on baseline CV risk–associated biomarker profiles through week 16 in patients with active PsA from the phase 2 study.
Methods: Patients (N = 203) were randomized 1:1:1 to placebo (n = 66), deucravacitinib 6 mg once daily (QD; n = 70), or deucravacitinib 12 mg QD (n = 67). Using 2 independent proteomic platforms, plasma samples from all patients with active PsA from the phase 2 PsA study with available biomarker data and 60 demographically matched normal healthy volunteers (NHVs) were analyzed to assess protein expression in patients with active PsA vs a healthy reference group and evaluate the impact of deucravacitinib on normalization of the expression levels of key proteins in patients with PsA at 16 weeks. SomaSignal CV risk score analyses were performed in 190 patients with PsA. Olink® Target 96 CVII and CVIII biomarker panels were used to investigate biomarkers relevant to CV risk in 203 patients with CV risk factors, including hypertension (HTN), and those without a history of known CV disease or HTN vs NHVs. Additionally, all patients were stratified by baseline Atherosclerotic Cardiovascular Disease (ASCVD) risk score (low [< 5%], borderline [5% to 7.4%], intermediate [7.5% to 19.9%], and high risk [≥ 20%]). Baseline data were analyzed using a pairwise Wilcoxon rank sum test. Olink® CV biomarker panels were analyzed based on a history of HTN or baseline ASCVD risk scores. A linear mixed-effects model was applied to evaluate the pharmacodynamic changes over time with deucravacitinib in all patients with PsA (serologic CV risk score analyses) and in patients stratified by history of HTN or ASCVD risk score (CV biomarker panels). Covariates included age, baseline body mass index, baseline disease activity (Psoriasis Area Severity Index [PASI] score and Disease Activity Score 28 [DAS28]), previous tumor necrosis factor (TNF) inhibitor use, and previous methotrexate use.
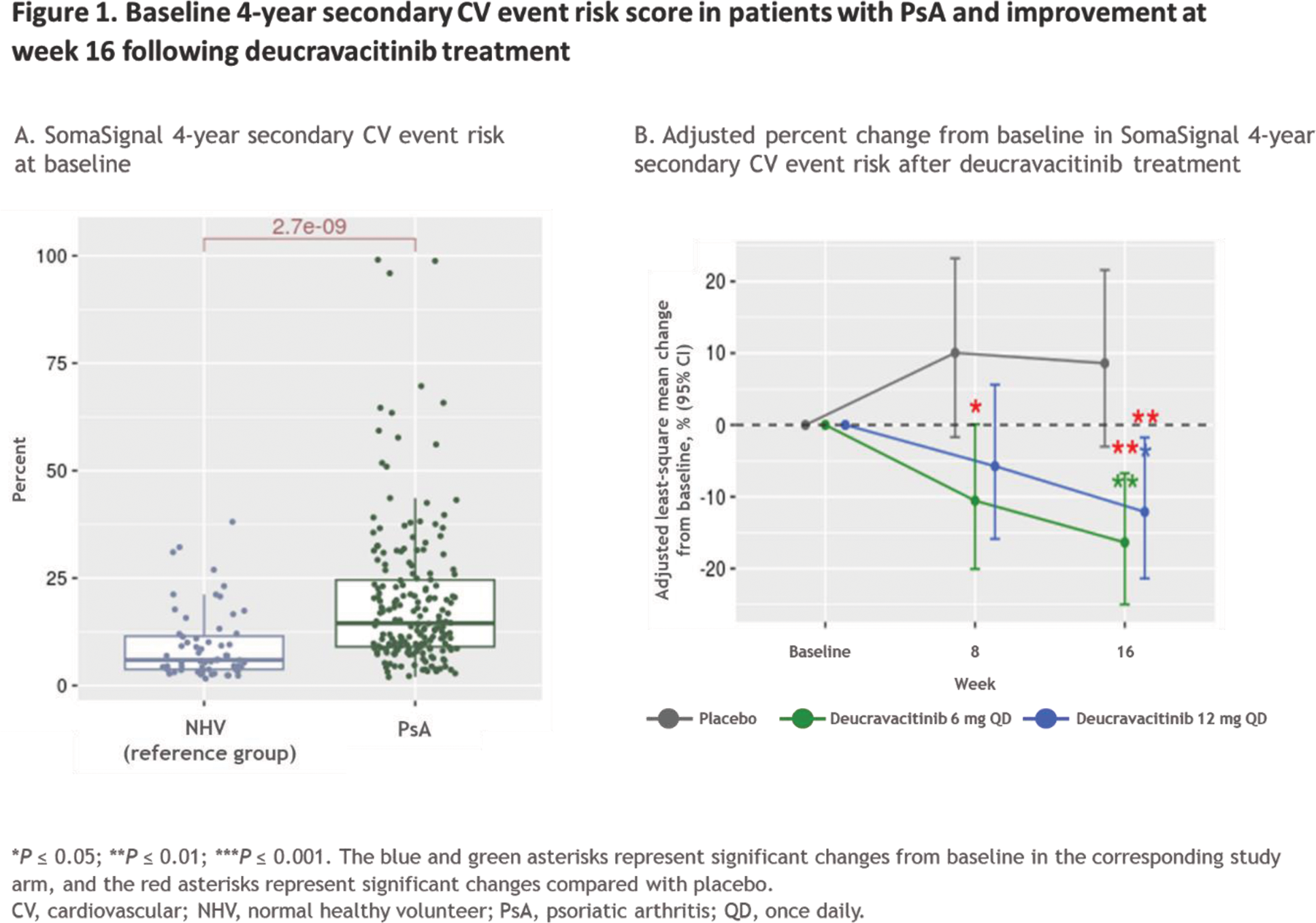
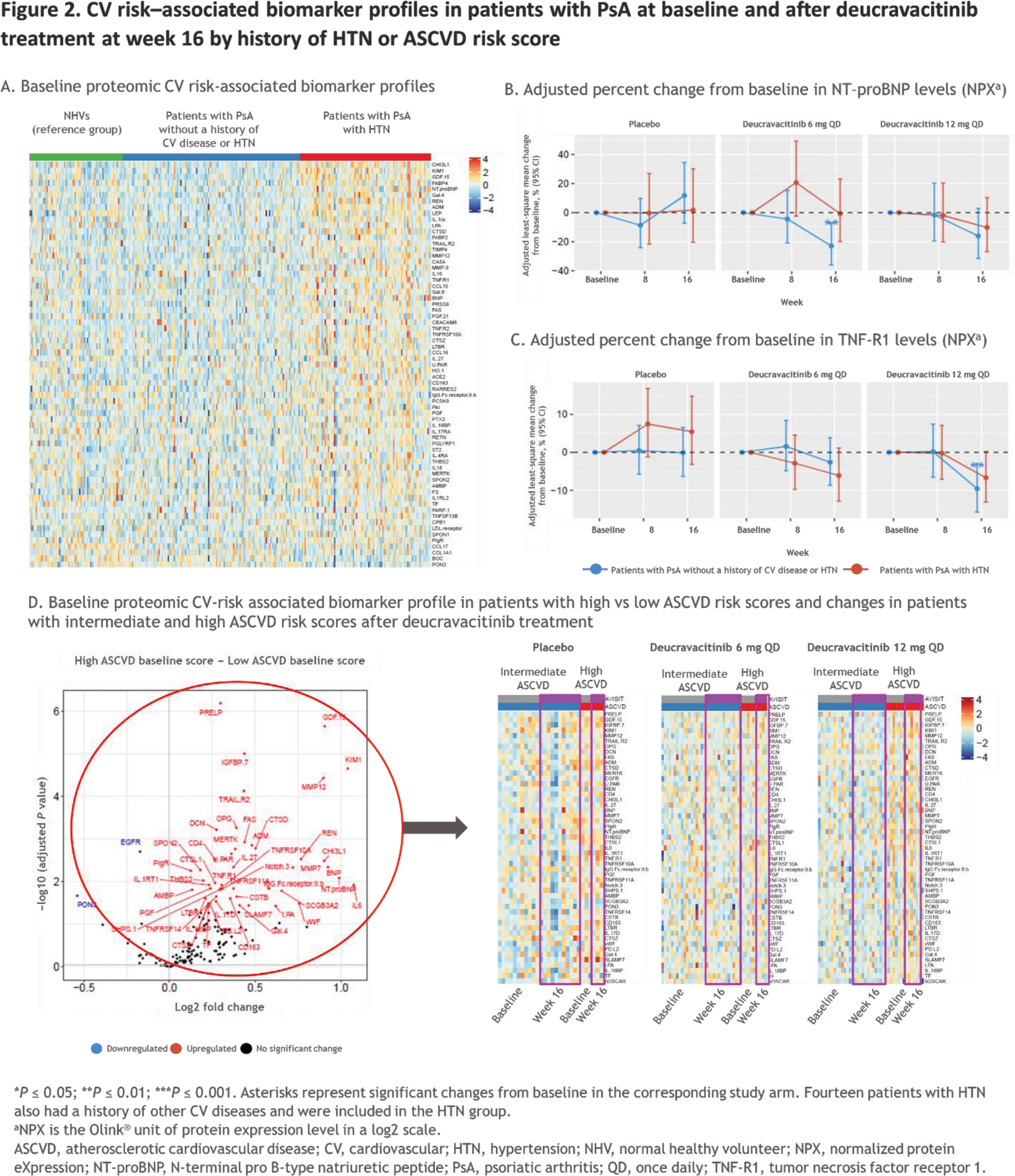
Results: In total, 84 patients had HTN and 103 patients did not have a history of known CV disease or CV risk factors including HTN. The distribution of 10-year risk score at baseline for ASCVD was 121 patients with low risk, 10 with borderline risk, 45 with intermediate risk, and 18 with high risk. In 190 patients with PsA, the SomaSignal CV risk tests, including cardiorespiratory fitness test and 4-year secondary CV event risk score, indicated lower cardiorespiratory fitness (ie, VO 2 max) scores and higher secondary CV risk scores at baseline vs the NHV reference group (Figure 1A). By week 16, a significant reduction in 4-year secondary CV event risk score was observed in patients treated with deucravacitinib compared with their baseline scores and compared with placebo (Figure 1B). Additionally, a significant improvement in cardiorespiratory fitness score was observed with deucravacitinib compared with baseline scores and placebo at week 16. CV risk – associated biomarkers from Olink ® CV panels were upregulated in patients with HTN compared with those without a history of CV disease or HTN and compared with the NHV reference group at baseline (Figure 2A). No change was observed with deucravacitinib treatment in patients with HTN; however, deucravacitinib improved CV risk – associated biomarker profiles, including decreases in NT-ProBNP (Figure 2B) and TNF-R1 (Figure 2C), in patients without a history of CV disease or HTN in a dose-dependent manner at week 16. When evaluating CV risk – associated biomarkers by ASCVD risk score, 49 CV risk – associated biomarkers were upregulated in patients with high versus low ASCVD risk scores at baseline. Further analysis of pharmacodynamic changes of these upregulated biomarkers in patients with intermediate or high ASCVD risk scores showed either no change or some normalization of these 49 biomarkers following treatment with deucravacitinib (Figure 2D).
Conclusion: Our findings suggest that treatment with deucravacitinib may improve serologic CV risk scores and CV risk–associated biomarker levels in patients with PsA. Differential proteomic profiles were observed in patients with a history of HTN and among patients with higher ASCVD risk scores; deucravacitinib treatment led to either no change or numerical improvement of the associated biomarker levels. Further evaluation of these observations is warranted in a larger PsA patient population from the ongoing phase 3 POETYK PsA-1 (NCT04908202) and POETYK PsA-2 (NCT04908189) studies as well as in other rheumatologic conditions with increased CV risk.
REFERENCES: [1] Polachek A, et al. Arthritis Care Res (Hoboken ) 2017;69:67–74.
[2] Vecellio M, et al. Front Immunol 2021;11:596086.
[3] Tsiogka A, et al. Biomedicines 2023;11:318.
Acknowledgements: We thank the patients and families who made this study possible, as well as the clinical teams that participated. The study was supported by Bristol Myers Squibb. All authors contributed to and approved the abstract; professional medical writing and editorial assistance was provided by Stephanie V. Koebele, PhD, of Nucleus Global, funded by Bristol Myers Squibb.
Disclosure of Interests: Christina Charles-Schoeman Octapharma, Boehringer Ingelheim, Recludix, Sana Biotechnology, Pfizer, AbbVie, Galapagos, and Bristol Myers Squibb, Bristol Myers Squibb, Priovant, CSL Behring, Janssen, Pfizer, and AbbVie, Brittany Weber Bristol Myers Squibb, Novo Nordisk, and Kiniksa Pharmaceuticals, Lu Gao Bristol Myers Squibb, Bristol Myers Squibb, Philip J. Mease AbbVie, ACELYRIN, Amgen, Bristol Myers Squibb, Century, Cullinan, Eli Lilly, Janssen, MoonLake Pharma, Novartis, Pfizer, and UCB, AbbVie, ACELYRIN, Amgen, Bristol Myers Squibb, Century, Cullinan, Eli Lilly, Janssen, MoonLake Pharma, Novartis, Pfizer, and UCB, AbbVie, ACELYRIN, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Josef S. Smolen AbbVie, Ananda, AstraZeneca, Astro, BMS, Celgene, Celltrion, Chugai-Roche, Galapagos, Immunovant, Janssen, Lilly, Sandoz, Pfizer, R-Pharma, Samsung, and Sanofi, AbbVie, Ananda, AstraZeneca, Astro, BMS, Celgene, Celltrion, Chugai-Roche, Galapagos, Immunovant, Janssen, Lilly, Sandoz, Pfizer, R-Pharma, Samsung, and Sanofi, AbbVie, AstraZeneca, Galapagos, and Lilly, Shangzhong Li Bristol Myers Squibb, Bristol Myers Squibb, Chun Wu Bristol Myers Squibb, Bristol Myers Squibb, Eleni Vritzali Bristol Myers Squibb, Bristol Myers Squibb, Peter Schafer Bristol Myers Squibb, Bristol Myers Squibb, Jinqi Liu Bristol Myers Squibb, Bristol Myers Squibb.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (