

Background: Tofacitinib was the first Janus kinase inhibitor (JAKi) approved by the US Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis (RA) in 2012. Since then, three additional members of the JAKi family, baricitinib, upadacitinib, filgotinib, have been approved for RA treatment. As important safety concerns have emerged, the regulatory authorities updated the recommendations for the utilization of JAKi. We aimed at studying how the prescription patterns of JAKi were impacted by the safety update.
Objectives: The objective of this study was to use a large collection of RA registries to assess how the rate of JAKi initiation and cessation changed over time with respect to the various safety warnings.
Methods: The JAK-pot collaboration is an observational study exploring the effectiveness and safety of various second line treatments, including JAKis and biologic DMARDs in adult RA patients. Data on individual treatment courses were supplied by ATTRA (Czech Republic), BIOBADASER (Spain), BIOREG (Austria), Biorx.si (Slovenia), GISEA (Italy), NOR-DMARD (Norway), REUMA.PT (Portugal), RHUMADATA (Canada), ROB-FIN (Finland), RRBR (Romania), SCQM (Switzerland), and UCRCR (Greece). Safety event and timeline considered. We considered 8 regulatory events related to safety warnings of JAKis: the first Safety Warning by the FDA (26 February 2019), the FDA Boxed Warning (26 July 2019), the EMA Confirmation of Caution (November 2019), first results from Safety Trials (end of January 2021), the FDA Warning on JAKis (February 2021), the presentation at the ACR Conference of Oral surveillance results (November 2021), the publication of the ORAL Surveillance Trial results (January 2022), and the EMA Recommendations for Specific Patient Groups (October 2022).
Statistical analysis: We calculated the percentage of patients on each type of treatment for each month from 2015 to end of 2023. For each of the 8 safety warnings, both JAKi initiation and cessation were modelled as a two-piece linear time trend within an 8-month time windows centred on the event. Logistic generalized estimating equations (GEE) with robust standard errors were used, clustering by patient. The regression was adjusted for baseline patient characteristics, baseline disease activity, treatment and disease characteristics.
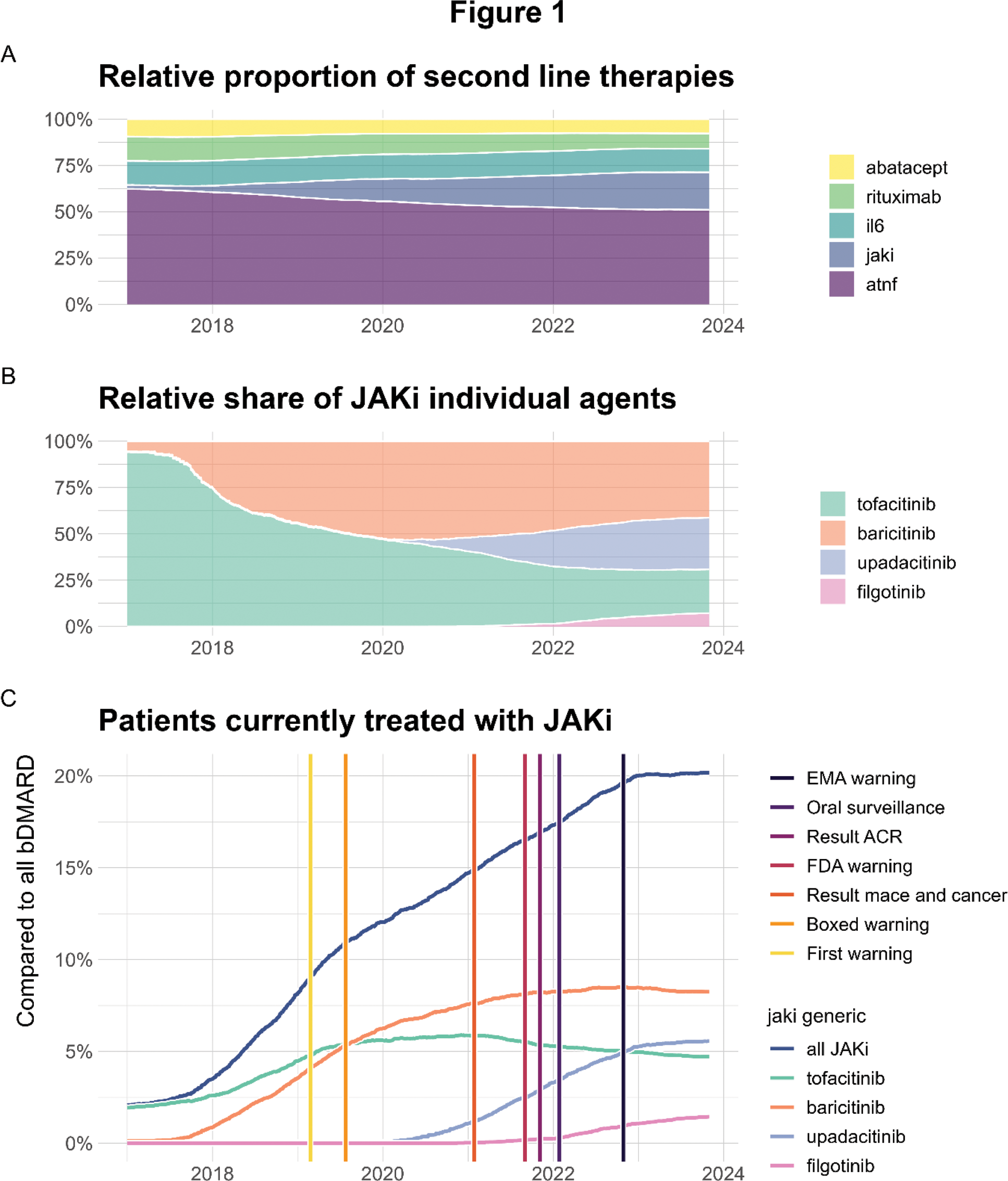
Results: Since 2015, 50,784 treatment courses have been recorded, involving 37,342 patients. Patients were predominantly female (75.5%), with a mean age of 57.6 years, and seropositive (77.1%). The proportion of patients on JAKi has increased from less than 1% in 2015 to around 20% in 2023 (Figure 1). From the beginning of 2015 until November 2017, the share of JAK inhibitor users rose by 0.6 percentage points (pp) per year. This rate accelerated significantly to 4.7 pp per year until July 2019, after which it slowed to an increase of 2.55 pp per year, to then level off in December 2022 to only 0.08 pp per year. Tofacitinib showed a first slowdown after the FDA Boxed Warning in July 2019 and a second one in November 2021, after the presentation of the ORAL-Surveillance trial results at the ACR Conference. Baricitinib experienced the most significant increase (2.74 pp/year) between October 2017 and the end of 2019, but then stabilized after August 2021. The more recently approved JAKi, upadacitinib and filgotinib, continued to grow, although upadacitinib growth rate started to decelerate in December 2022.
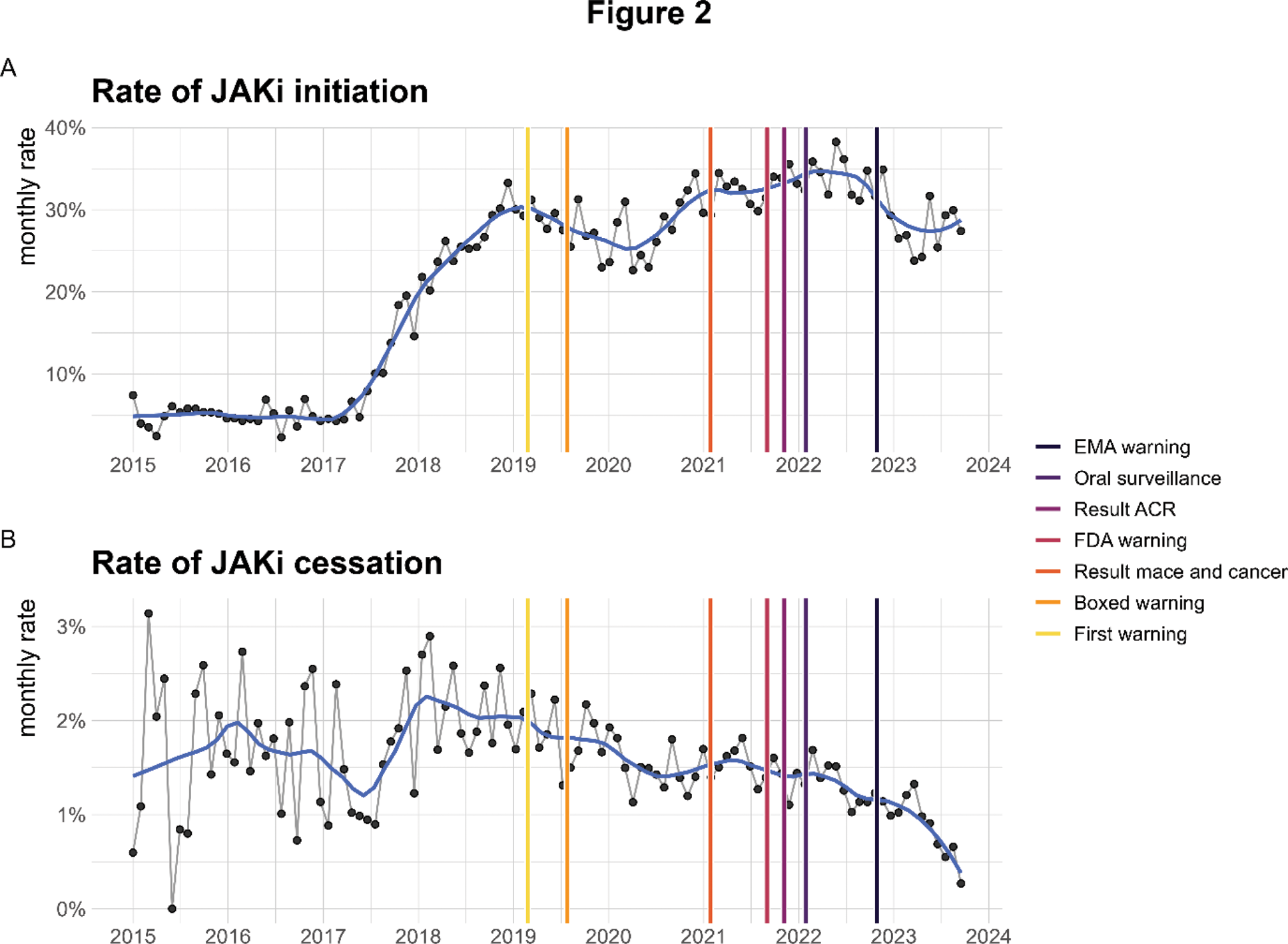
JAKi treatment initiation: The monthly rate of JAKi initiation showed a clear increase between mid-2017 and beginning of 2019, reaching approximately 30% of second line initiations and subsequently fluctuating between 25% and 35% (Figure 2). Adjusted analyses indicated significant decrease around the first warning and the first results from the safety trial, with the initiation rate declining by 13.8 [4.7 – 22.9] and 13.1 [3.7 – 22.4] pp respectively. The first change was primarily a decline in baricitinib initiations, (-10.16 [-17.6; -2.8] pp per year), the second was mainly a decrease of tofacitinib initiation rates. Upadacitinib initiations also declined after the presentation of the ORAL Surveillance trial findings at the ACR Conference and its subsequent publication.
JAKi treatment discontinuation: The monthly rate of discontinuation of JAKi declined from around 2% in 2018 to less than 1% by 2023. Adjusted piecewise regression revealed a slight increase of JAKi discontinuations of 0.8 pp per year after the “ACR results”, that was primarily driven by treatment stops of upadacitinib. The discontinuation rate for baricitinib increased by 1.4 [0.2, 2.5] pp per year after the “FDA warning.”
Conclusion: In this large international collaboration of registries, we found that the relative use of JAKi had increased from 1% to 20% over 9 years, with two clear slowdowns after the first safety FDA and EMA warnings. The first slowdown was mainly driven by a decrease of baricitinib and tofacitinib initiations, the second by a decrease in upadacitinib initiations. The use of tofacitinib also decreased after the publication of the Oral surveillance trial findings because of a decrease of drug initiation, but the trend on JAKi use was compensated by the global increase of upadacitinib. The global plateau of baricitinib use at the end of 2021 seems to be linked to an increase in its discontinuation following the first FDA warning. In conclusion, the safety warnings did affect the prescription pattern of JAKi in patients with RA, with a substantial decrease in initiation of tofacitinib and baricitinib, and to a lesser extent of upadacitinib.
Funding: This study is investigator initiated. The JAK-pot collaboration is supported by unconditional/unrestricted research grants from Pfizer Inc, Galapagos NV, AbbVie Inc., Eli Lilly and Co., and Alfasigma S.p.A.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Denis Mongin: None declared, Romain Aymon: None declared, Denis Choquette: None declared, Louis Coupal: None declared, Catalin Codreanu: None declared, Florenzo Iannone Abbvie, Alfasigma, Amgen, Astra-Zeneca, Csl-Vifor, GSK, Janssen, Novartis, Lilly, UCB, Abbvie, Amgen, Astra-Zeneca, Janssen, Lilly, UCB, Sella Aarrestad Provan: None declared, Ruth Fritsch-Stork: None declared, Tore K. Kvien Grünenthal, Janssen, Sandoz, AbbVie, Gilead, Janssen, Novartis, Pfizer, Sandoz, UCB, Dan Nordström MSD, Novartis, Pfizer, UCB, Karel Pavelka AbbVie, Eli Lilly, Sandoz, UCB, Medac, Pfizer, Jakub Závada Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Sanofi, AstraZeneca, Sobi, Abbvie, Novartis, AstraZeneca, Glaxo, Manuel Pombo-Suarez: None declared, Lucía Otero-Varela: None declared, Elsa Vieira-Sousa: None declared, Ziga Rotar Abbvie, Amgen, AstraZeneca, Boehringer, Biogen, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sandoz Lek, Stada, SOBI, Abbvie, AstraZeneca, Boehringer, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sandoz Lek, SOBI, Prodromos Sidiropoulos: None declared, Antonios Bertsias: None declared, Delphine Sophie Courvoisier: None declared, Axel Finckh AstraZeneca, Eli-Lilly, GlaxoSmithKline, MSD, Pfizer, Pfizer BMS AbbVie Galapagos NV Eli-Lilly, Kim Lauper Pfizer, Ori Elkayam: None declared, Victoria Furer: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (