

Background: There is an increasing interest in adding artificial intelligence (AI) and robots to routine care in order to optimize the clinical resources. Early diagnosis is crucial in rheumatoid arthritis (RA) for achieving optimal treatment outcomes. However, limited access to ultrasound specialists can delay diagnosis, underscoring the need for feasible, automated systems for synovitis assessment to improve arthritis diagnosis. The Arthritis Ultrasound Robot (ARTHUR) is an innovative CE-marked automated system designed to perform ultrasound scans of hand and wrist joints. When integrated with the automatically AI scoring model, DIANA, it provides an efficient method for assessing and scoring synovitis.
Objectives: To evaluate the diagnostic performance of robotic ultrasonography (RUS) as compared to Human ultrasonography (HUS) performed by the rheumatologist for assessing synovitis in the wrist and finger joints in healthy controls and patients with rheumatoid arthritis (RA).
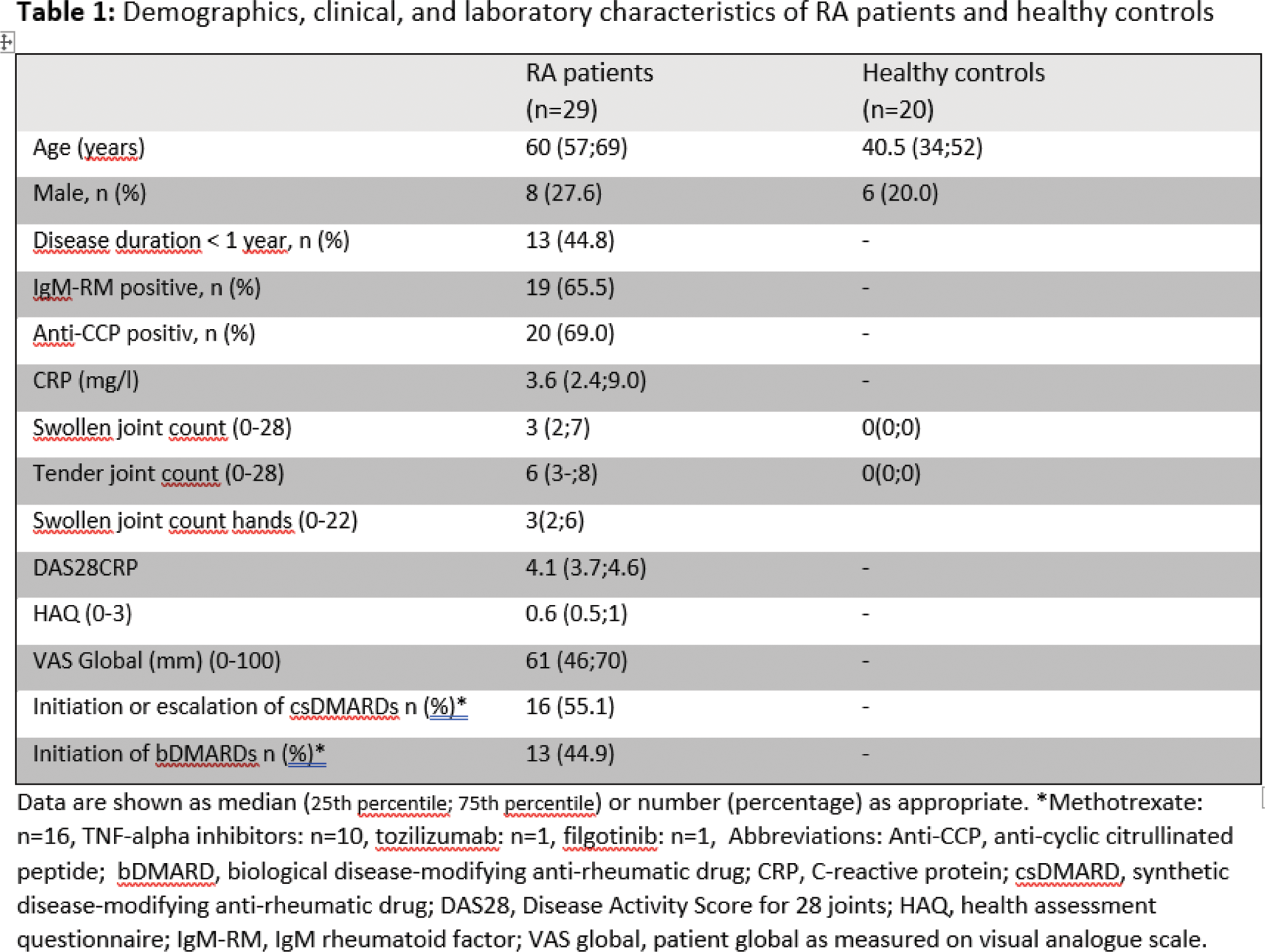
Methods: Twenty healthy controls, recruited from hospital staff, with no clinically swollen or painful joints in the hands, and 29 RA patients eligible for initiation or intensification of conventional synthetic or biological disease-modifying anti-rheumatic drugs (DMARDs), with at least one clinically swollen joint in the hand, were included (Table 1). The ultrasound assessment included the wrists (intercarpal position), 1st-5th metacarpophalangeal joints (MCPs), 1st interphalangeal joint (IP), and 2nd-4th proximal interphalangeal joints (PIPs) in both hands using RUS and HUS. ARTHUR scanned the controls and patients twice without interval for assessing intra-robot reliability. Synovitis was scored in the standard position from 0-3 for Grey Scale (GS) and Colour Doppler (CD) using the EULAR_OMERACT Synovitis Scoring system. The RUS images obtained by ARTHUR were scored by the DIANA software AI model. The HUS images were scored by an experienced reader who had demonstrated high intra- and inter-reader agreement for the scoring system (quadratic weighted Kappa: 0.88–0.95) but was not blinded to the clinical diagnosis. The total score range was 0–66. The Disease Activity Score-28 (DAS28), health assessment questionnaire, patient global visual analogue scale and 28-joint swollen and tender joint count (SJC and TJC) were assessed at inclusion for RA patients. Additionally, a 22-joint “SJC-hands” corresponding to the joints examined by ultrasound was evaluated for both healthy controls and RA patients. Descriptive statistics and agreement were evaluated for examinations by RUS1 vs. RUS2 (intra-robot agreement) and between RUS (RUS1/RUS2) and SUS (inter reader agreement) for the sum scores of all joints. Sensitivity and specificity were calculated for true synovitis, defined as at least one joint out of 22 with GS > 1 or GS = 1 combined with CD > 1. Agreement for dichotomous outcomes was assessed using Cohen’s kappa, while single measure intraclass correlation coefficients (ICCs) were used to evaluate continuous outcomes.
Results: Characteristics of patients and controls
Twenty healthy controls (14 females, 6 males) and 29 RA (21 females, 8 males) patients were included. Patients initiated bDMARDs or initiated or escalated csDMARDs. Demographics and inclusion characteristics are presented in Table 1. The median (25th percentile; 75th percentile) age for RA patients were 60 years (57;69) while for healthy controls 40.5 years (34;52).
Diagnostic accuracy, sensitivity, and specificity
The diagnostic sensitivity and specificity of human US were 100% and 100%, respectively. For the first robotic US (RUS1), a sensitivity of 89% and a specificity of 5% were observed. The agreement with human US was 54% as measured by accuracy and 0.06 as measured by Kappa. Similarly, RUS2 showed a sensitivity of 90% and a specificity of 0%, with an agreement with SUS HUS of 52% and a Kappa value of 0.04.
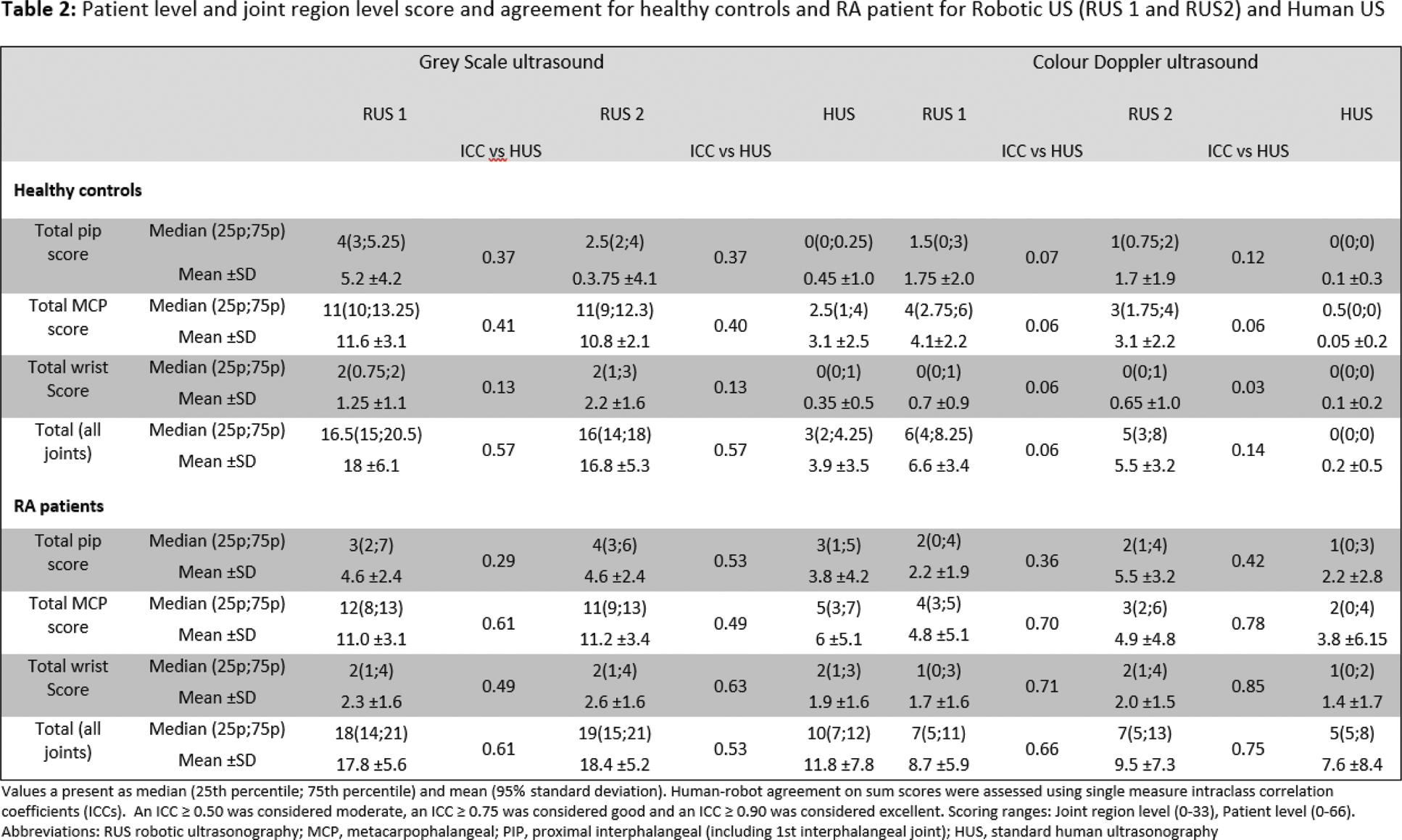
Sum scores in controls and patients (patient level)
Median (25th percentile; 75th percentile) sum scores for healthy controls’ joints were for RUS1: GS 16.5 (15; 20.5), CD 6 (4; 8.25); for RUS2: GS 16 (14; 18), CD 5 (3; 8); and for HUS: GS 3 (2; 4.25), CD 0 (0; 0), with moderate agreement for GS (ICC 0.57). Corresponding values for RA patients were RUS1: GS 18 (14; 21), CD 7 (5; 13); for RUS2: GS 19 (15; 21), CD 7 (5; 13); and for HUS: GS 10 (7; 12.5), CD 5 (3; 8) (Table 2).
Conclusion: This study demonstrated high sensitivity but low specificity for detecting synovitis using robotic US. Alternative cutoff values for true synovitis may be considered. Robotic US overestimated GS scores in the MCP region for both healthy controls and RA patients, which limited its diagnostic performance. Despite these limitations, robotic US shows promise, particularly at the wrist level. Further development and software adjustments may be needed to improve its overall diagnostic accuracy.
REFERENCES: NIL.
Acknowledgements: To our fantastic project nurses, Anne-Mette, Bettina, and Charlotta, who have made an invaluable contribution.
Disclosure of Interests: Mads Ammitzbøll-Danielsen ROPCA ApS provided Arthur free of charge and supported the study through an unrestricted research grant, without access to the data or involvement in data analysis or manuscript preparation, Mikkel Østergaard Abbvie, BMS, Celgene, Eli-Lilly, Galapagos, Gilead, Janssen, MEDAC, Merck, Novartis, Pfizer, Sandoz, and UCB, Abbvie, BMS, Merck, Novartis and UCB, Ho Pui Lydia Tam: None declared, Lene Terslev Novarits, UCB, Johnson and Johnson and GE Healthcare.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (