

Background: TYK2 inhibitors (TYK2i) have shown efficacy in treating psoriasis and are being investigated for other immune-mediated diseases. While TYK2i are known to suppress multiple inflammatory signaling pathways, including Th17 pathways, their effects on other immune cells remain incompletely understood.
Objectives: This study aimed to elucidate the mechanism of action of TYK2i in spondyloarthritis (SpA) utilizing an experimental animal model and human samples from psoriasis patients with arthritis/enthesitis undergoing TYK2i treatment.
Methods: SKG mice were used as a SpA model. TYK2i (BMS986202) (30 mg/kg qd) was orally administered after induction of arthritis, dermatitis, and spondylitis by curdlan. Immune cells were analyzed by flow cytometry, immunohistostaining, and fixed single-cell RNA sequencing analysis. Neutrophil heterogeneity and NETosis were evaluated by cell surface markers and MPO/citrullinated histone staining, respectively, both in SKG mice and psoriasis patients.
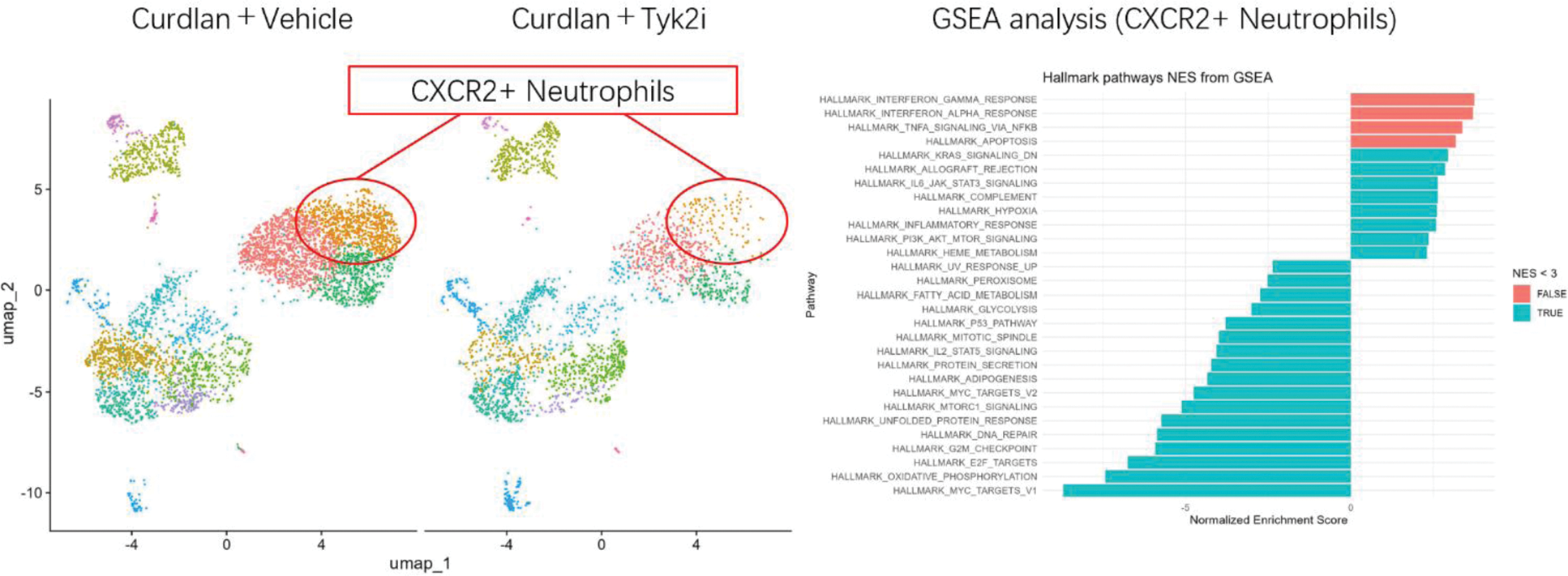
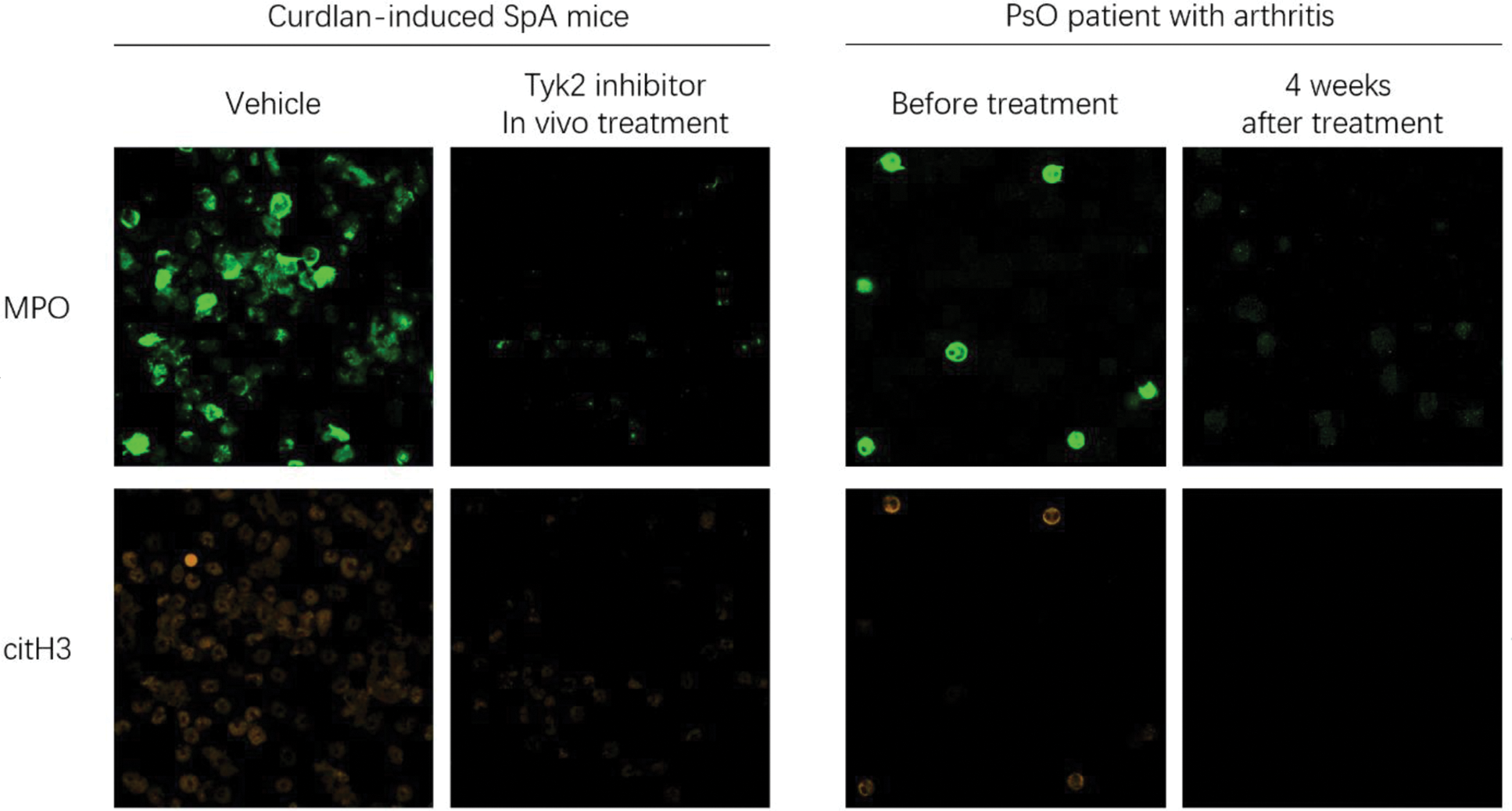
Results: Early treatment with TYK2i rapidly inhibited arthritis and dermatitis in SKG mice. Neutrophils heavily expanded in the bone marrow and inflammatory tissues of SKG mice. TYK2i reduced Th17 numbers, but the suppression of neutrophils by TYK2i occurred well before the reduction of Th17 cells, suggesting a direct effect of TYK2i on neutrophils. Single-cell analysis of synovial tissue revealed that neutrophils were predominantly regulated by type-I interferon signaling and NETosis pathways. These neutrophils expressed multiple cytokine and chemokine receptors, including IL-17RA and CXCR2, which were suppressed by TYK2i. Interferon-related neutrophils were already present in the bone marrow of SKG mice, and NETosis was significantly inhibited by TYK2i. TYK2i also inhibited NETosis in blood samples from psoriasis patients with arthritis/enthesitis undergoing TYK2i treatment.
Conclusion: This study uncovered a previously unknown mechanism of action of TYK2i on neutrophils and NETosis in SpA. The findings support and extend the potential effectiveness of TYK2i for SpA and other inflammatory diseases such as SLE and ANCA-associated vasculitis, where neutrophils and NETosis play a role in disease initiation.
TYK2 inhibitor suppresses Type 1 Interferon signature in neutrophils .
Tyk2 inhibitor suppresses Neutrophil Extracellular Traps.
Acknowledgements: I sincerely appreciate all who contributed to this research.
Disclosure of Interests: Masao Katsushima: None declared , Hongxin Sun: None declared , Yuhei Fujisawa: None declared , Kazuo Fukumoto: None declared , Ryu Watanabe: None declared , Shinsuke Yamada: None declared , Katsuyoshi Habiro Bristol Myers Squibb, Naonobu Sugiyama Bristol Myers Squibb, Motomu Hashimoto Bristol Myers Squibb.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (
REFERENCES: NIL.