

Background: Ankylosing Spondylitis (AS) is an auto-inflammatory disease, which is characterized by chronic inflammation of the spine, sacroiliac and occasionally peripheral joints. IL-17A inhibitors have emerged as a key class of biologics for AS treatment. However, approximately 40% of AS patients didn’t respond to IL-17A inhibitors. A recent study utilizing flow cytometry to analyze CD4+ T cell subsets identified an enhanced type I interferon (IFN) signature in non-responders, suggesting a potential mechanism for treatment resistance. However, identifying the molecular mechanisms of IL-17A inhibitors and predicting patient responses remains challenging when relying solely on single-omics studies. Therefore, we first performed the inaugural multi-omics analysis focused on IL-17A inhibitor therapy for AS, aiming to decipher these pharmacological mechanisms.
Objectives: To elucidate the pharmacological mechanisms of IL-17A inhibitors in the treatment of ankylosing spondylitis (AS) and to investigate the reasons behind the lack of response in some AS patients to IL-17A inhibitors.
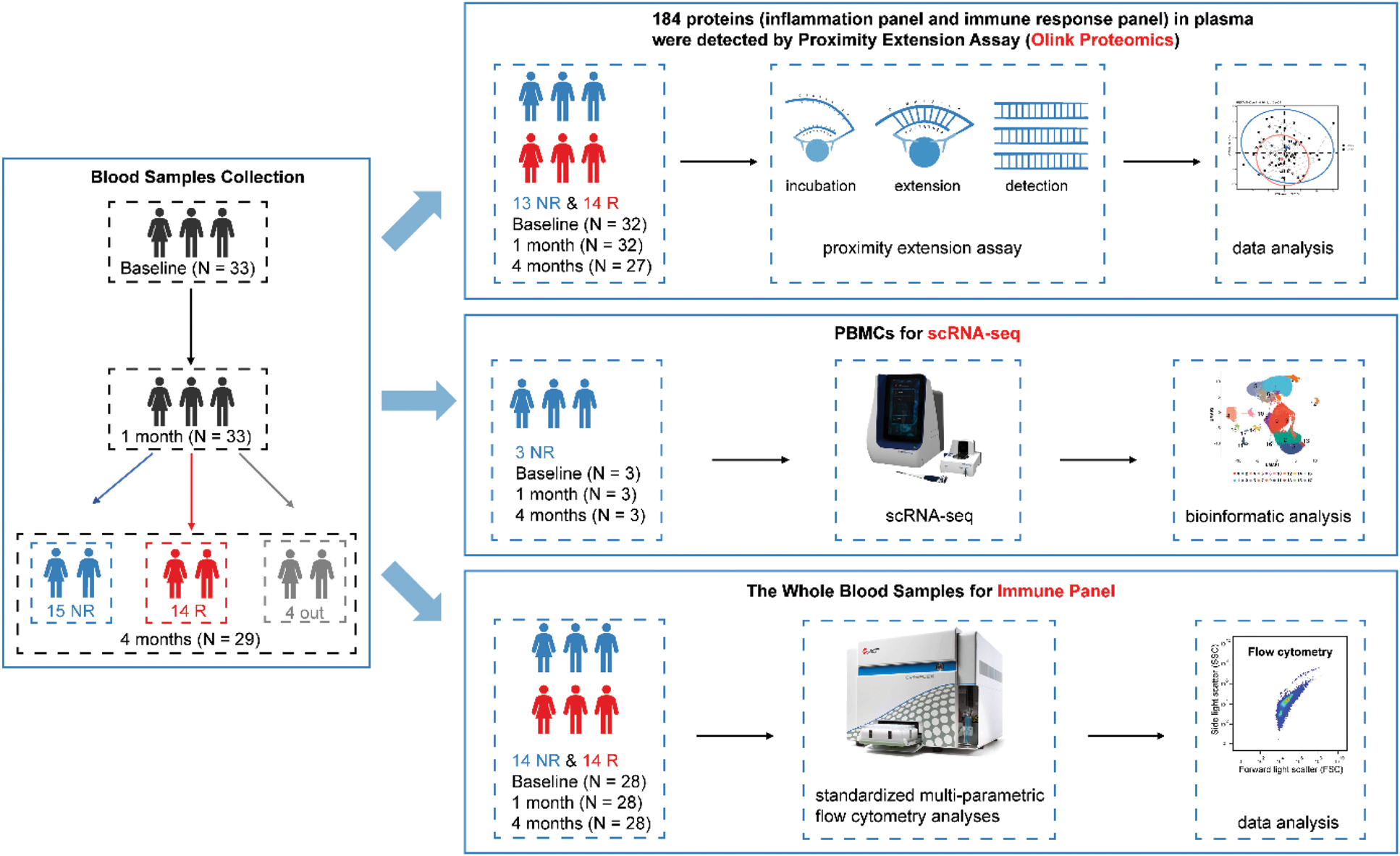
Methods: We collected blood samples from AS patients before treatment with IL-17A inhibitors and at approximately 1 month and 4 months following treatment initiation (Figure 1). We analyzed plasma samples using Olink proteomics, detecting 184 proteins from the inflammation and immune response panels through the Proximity Extension Assay (PEA). After quality control, there were 70 proteins in the inflammation panel and 80 proteins in the immune response panel available for subsequent analysis. Moreover, the Peripheral Blood Mononuclear Cells (PBMCs) were isolated for single cell RNA-seq (scRNA-seq). Furthermore, standardized multi-parametric flow cytometry analyses, also referred to as the Immune Panel, were performed on fresh whole blood samples. In addition, we utilized the Olink proteomics data from the UK Biobank (UKB), which included 2923 plasma proteins from 53,014 participants, to cross-validate our research findings. We performed the cross-sectional study using logistic regression analysis and conducted the longitudinal study by Cox proportional hazards regression analysis.
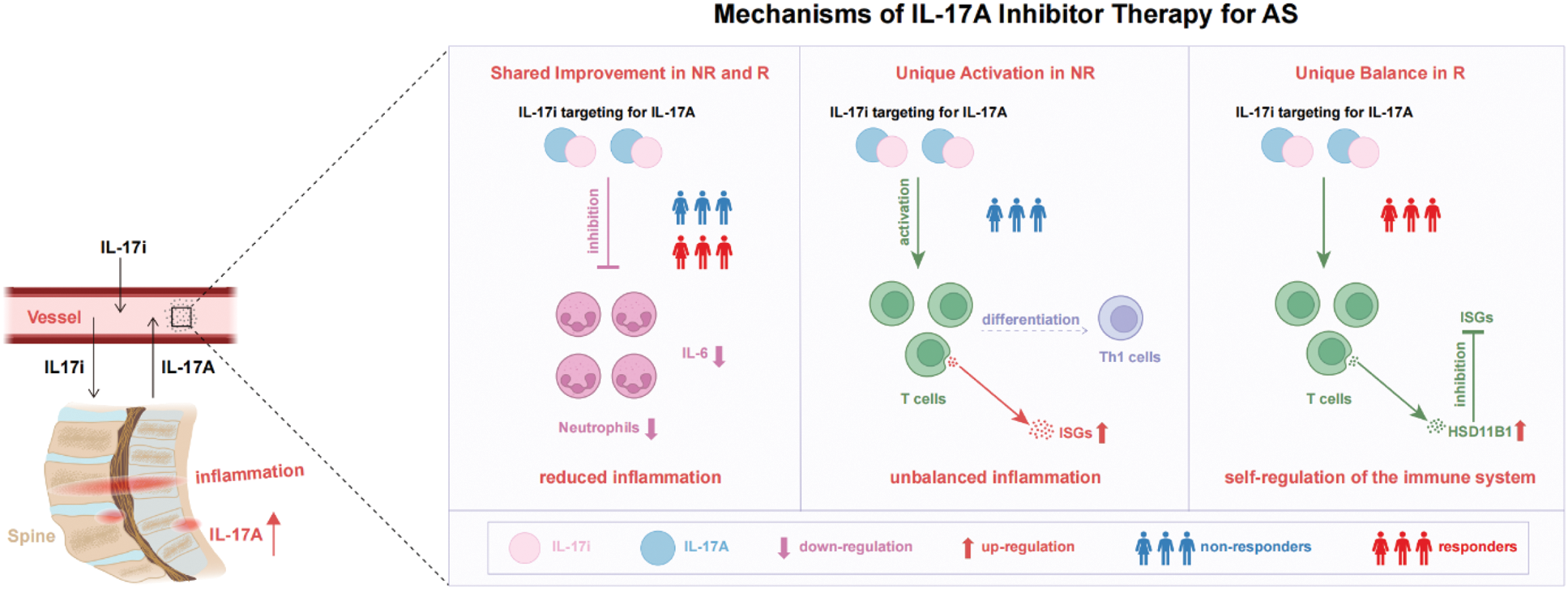
Results: Proteomic profiling was altered by IL-17A inhibitor treatment, with 59 differentially expressed proteins (DEPs) identified before and after treatment. Among these, 21 proteins were downregulated, while 38 were upregulated. These proteins were enriched in pathways such as IL-17 signaling, TNF signaling and JAK-STAT signaling. Among these, the enrichment of the JAK-STAT signaling pathway persisted across all three periods. Additionally, changes in the proteins involved in the JAK-STAT signaling pathway showed the strongest correlation with changes in clinical parameters, compared to those in the IL-17 or TNF signaling pathways. Furthermore, we found that the up-regulation of Interferon-Stimulated Genes (ISGs) were the main contributing factor to the lack of response to IL-17A inhibitors by scRNA-seq analysis. Correspondingly, the AUC values of IFN or JAK-STAT related proteins—LAMP3 and HSD11B1—were 0.857 and 0.813 respectively. Remarkably, our study coincidentally included a patient with both AS and Sicca syndrome (SS). In contrast to his repeatedly fluctuating clinical scores during the treatment of IL-17A inhibitors, his clinical scores have been maintained at a low inflammatory level over the long term after switching to JAK-STAT inhibitors.
Conclusion: AS patients with abnormalities in IFN or JAK-STAT-related pathways, such as those with both AS and SS, are not suitable candidates for IL-17A inhibitor therapy. These related proteins, including LAMP3 and HSD11B1, hold potential as powerful predictors of therapeutic response. Our findings are expected to guide more precise medication recommendations for AS patients, advancing personalized treatment strategies.
The pipeline of our study. NR, non-responders. R, responders.
The mechanisms of IL-17A inhibitor therapy for AS. AS, Ankylosing Spondylitis. NR, non-responders. R, responders. IL-17i, IL-17A inhibitor.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (