

Background: The coexistence of obesity and osteoporosis exemplifies the intricate communication and coordination between adipose and bone tissues [1]. Rheumatoid arthritis, a chronic inflammatory joint disorder, can lead to damage of cartilage and bone, resulting in disability. Adiponectin, which is secreted by adipocytes, plays a crucial role in regulating osteoblast formation and function, thereby influencing bone mass through both direct and indirect pathways [2]. A novel mesenchymal subpopulation known as ‘marrow adipogenic lineage precursors’ (MALPs) has been identified, characterized by the expression of various adipocyte markers while lacking lipid droplets [3]. Serving as precursors for lipid-laden adipocytes, MALPs are predominantly found as pericytes and stromal cells, creating a comprehensive three-dimensional network within the marrow cavity. Nevertheless, the precise mechanisms by which MALPs regulate bone and joint homeostasis under both physiological and pathological conditions remain elusive. Sclerostin serves as a significant inhibitor of bone formation, antagonizing Wnt/β-catenin signaling [4]. Prior research has demonstrated that the use of neutralizing monoclonal antibodies against sclerostin has been shown to enhance bone mass and is being explored as a treatment for osteoporosis. However, it might increase the risk of cardiovascular diseases, potentially because sclerostin plays a role in protecting cardiovascular system [5]. Conditional ablation of sclerostin in MALPs might increase bone mass and promote bone and joint homeostasis, while ameliorating the systemic side effects.
Objectives: We ought to explore the contribution of MALP-derived sclerostin in control of bone and joint homeostasis by conditionally ablating of Sost gene, which encodes sclerostin, using the Adipoq-Cre system that predominantly targets MALPs. We are also investigating the potential of targeting Adipoq + Sost + cells in the bone marrow as an innovative therapeutic approach for the treatment of osteoporosis and rheumatoid arthritis.
Methods: We assessed the bone mass of conditional knockout (cKO) mice and control mice at various ages utilizing micro-computed tomography (μ-CT), followed by histological analyses. Additionally, we evaluated the osteogenic potential of bone marrow stromal cells (BMSCs) derived from both cKO and control mice in vitro . Finally, we established two animal disease models: the ovariectomy-induced osteoporosis model and the collagen antibody-induced arthritis model, measuring the bone and joint parameters across both groups.
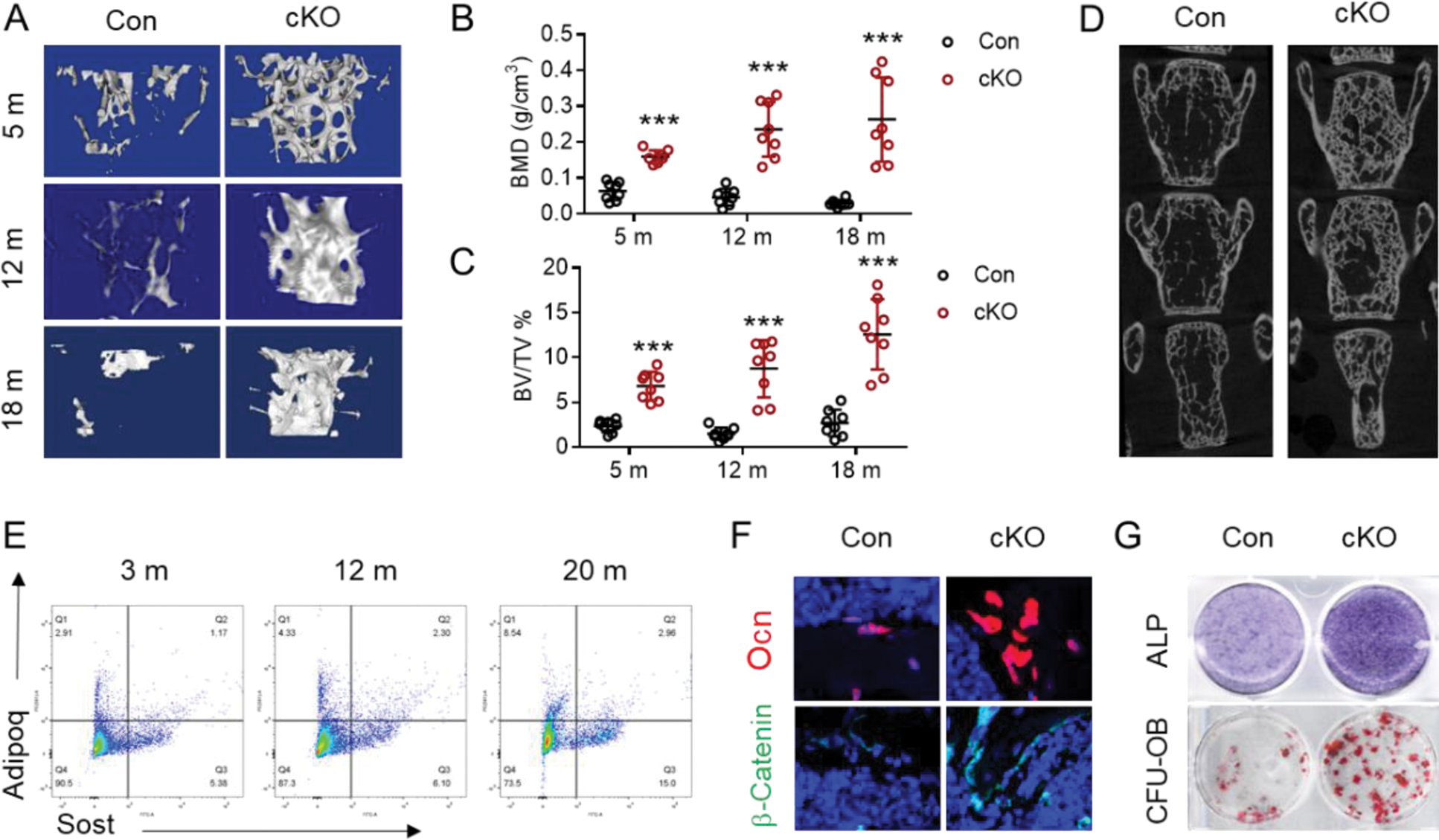
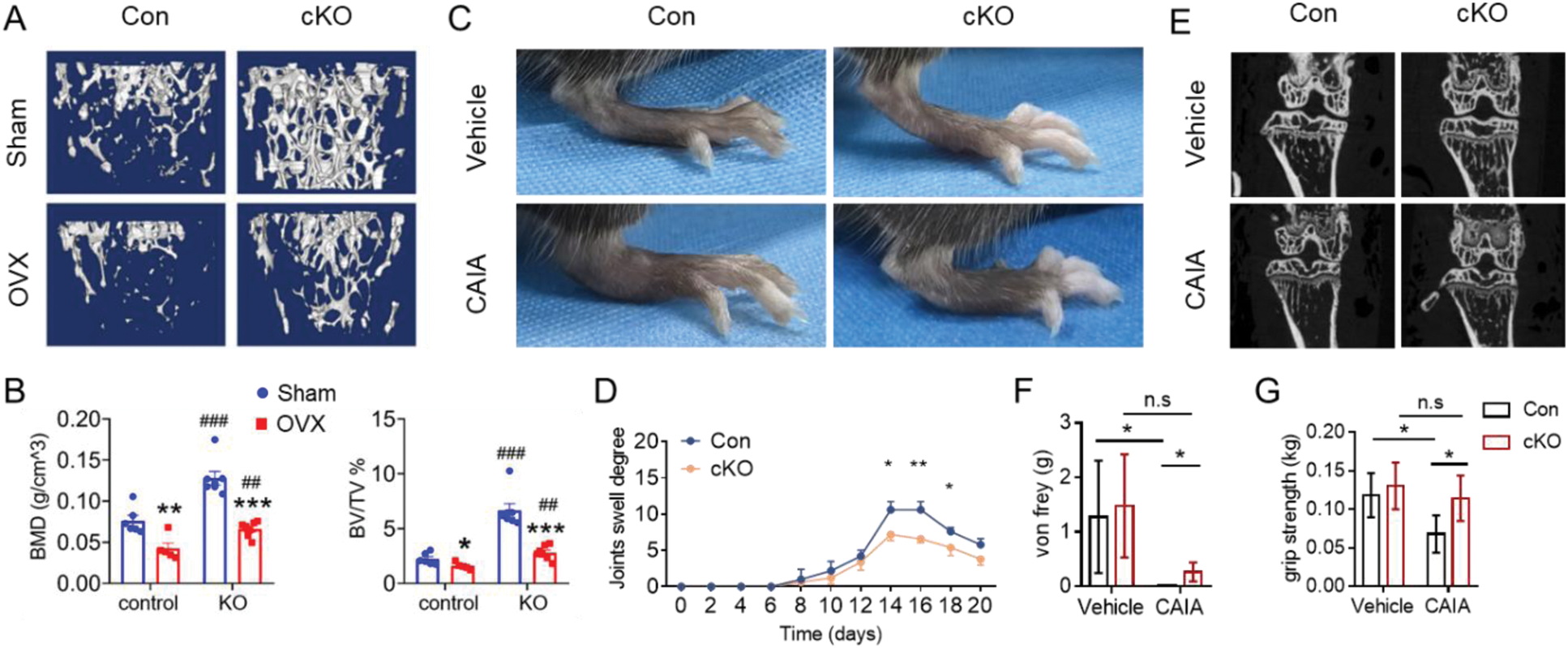
Results: Our investigation revealed that mice lacking sclerostin within the MALPs (Sost fl/fl ; Adipoq Cre , cKO) exhibited no significant alterations in peripheral adipose tissue mass or glucose metabolism. μCT analysis of the skeletal structure indicated that cKO mice demonstrated an increase in bone mass at 5 months of age, particularly within the female group (Figure 1A-C). This phenotype became increasingly pronounced with advancing age (12 and 18 months). Notably, the spinal bone mass remained unaffected by sclerostin deletion at 5 months, yet showed a significant increase at 18 months in cKO mice (Figure 1D). Simultaneously, we observed an increase in the proportion of Adipoq + Sost + cells as the mice aged (Figure 1E). Immunofluorescence staining revealed a higher presence of osteoblasts surrounding the trabecular regions, along with an elevated expression of active-β-catenin protein in the bone of cKO mice compared to controls (Figure 1F). The levels of osteogenic marker proteins were markedly enhanced in primary BMSC cultures derived from cKO mice relative to those from control mice (Figure 1G). Subsequently, we assessed the impact of sclerostin deficiency in mice subjected to ovariectomy and a collagen antibody-induced arthritis model. Our findings indicated that the deletion of Sost in MALPs partially mitigated the osteoporotic characteristics associated with estrogen deficiency (Figure 2A, B). Additionally, Sost deletion alleviated the symptoms of collagen antibody-induced arthritis, including ameliorated joint swell (Figure 2C, D), bone erosion (Figure 2E), mechanical pain threshold (Figure 2F), and grip strength (Figure 2G).
Conclusion: We establish that the Adipoq + Sost + cell subpopulation within the bone marrow plays a vital role in the BMSC osteogenic differentiation. This distinct cell population in the bone marrow could serve as a valuable target for therapeutic interventions in osteoporosis and rheumatoid arthritis.
REFERENCES: [1] Sebo ZL, Rendina-Ruedy E, Ables GP, et al. Bone Marrow Adiposity: Basic and Clinical Implications. Endocr Rev . 2019;40(5):1187-1206.
[2] Zou W, Rohatgi N, Brestoff JR, et al. Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain. Cell Metab . 2020;32(5):801-813.e6.
[3] Zhong L, Lu J, Fang J, et al. Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone. Elife . 2023;12:e82112.
[4] Eriksen EF, Chapurlat R, Boyce RW, et al. Modeling-Based Bone Formation After 2 Months of Romosozumab Treatment: Results From the FRAME Clinical Trial. J Bone Miner Res . 2022;37(1):36-40.
[5] Zheng J, Wheeler E, Pietzner M, et al. Lowering of Circulating Sclerostin May Increase Risk of Atherosclerosis and Its Risk Factors: Evidence From a Genome-Wide Association Meta-Analysis Followed by Mendelian Randomization. Arthritis Rheumatol . 2023;75(10):1781-1792.
Bone marrow Adipoq + Sost + cell subpopulation plays an important role in bone formation and BMSC osteogenic differentiation.
Depletion of Sost in MALPs ameliorates osteoporosis and rheumatoid arthritis in mice.
Acknowledgements: This work was supported, in part, by the National Natural Science Foundation of China Grants (82430078, 82261160395, 82230081, 82004395, 82302767).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (