

Background: Systemic sclerosis (SSc) is an autoimmune disease characterized by type I interferon (IFN-I) production and mitochondrial dysfunction. Emerging evidence suggests that activation of the stimulator of interferon genes (STING) pathway in SSc is triggered by cytosolic mitochondrial DNA (mtDNA) released during mitochondrial stress. This link is further supported by the similarities between SSc and SAVI (STING-associated vasculopathy with onset in infancy) syndrome, a condition caused by a gain-of-function mutation in TMEM173 (which encodes STING) that phenocopies many key features of SSc. Nevertheless, the role of mitochondrial damage in activating cGAS-STING and driving IFN-I production in SSc remains poorly understood.
Objectives: To explore the contribution of mitochondrial dysfunction to cGAS-STING pathway activation and its role in IFN-I production in SSc.
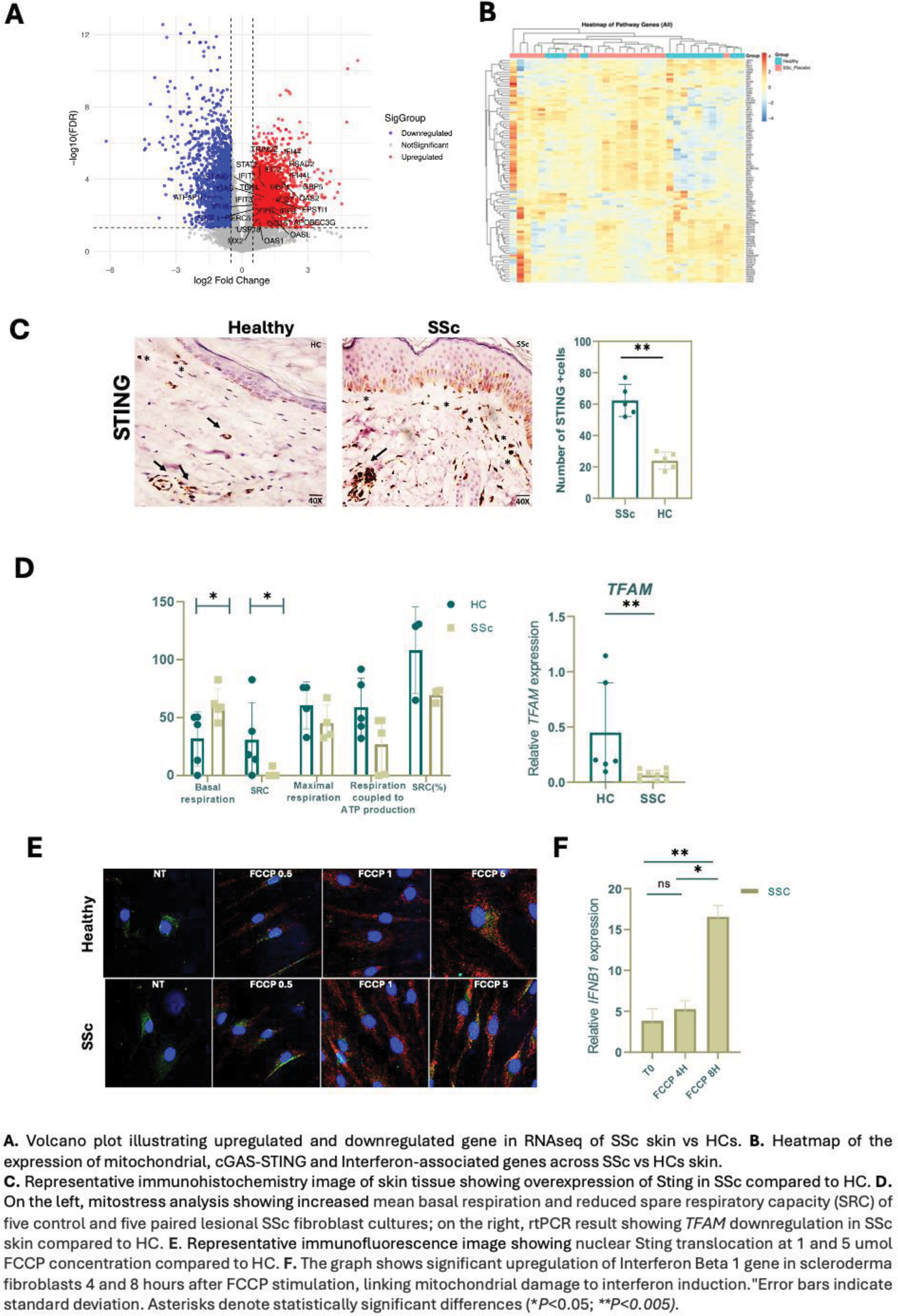
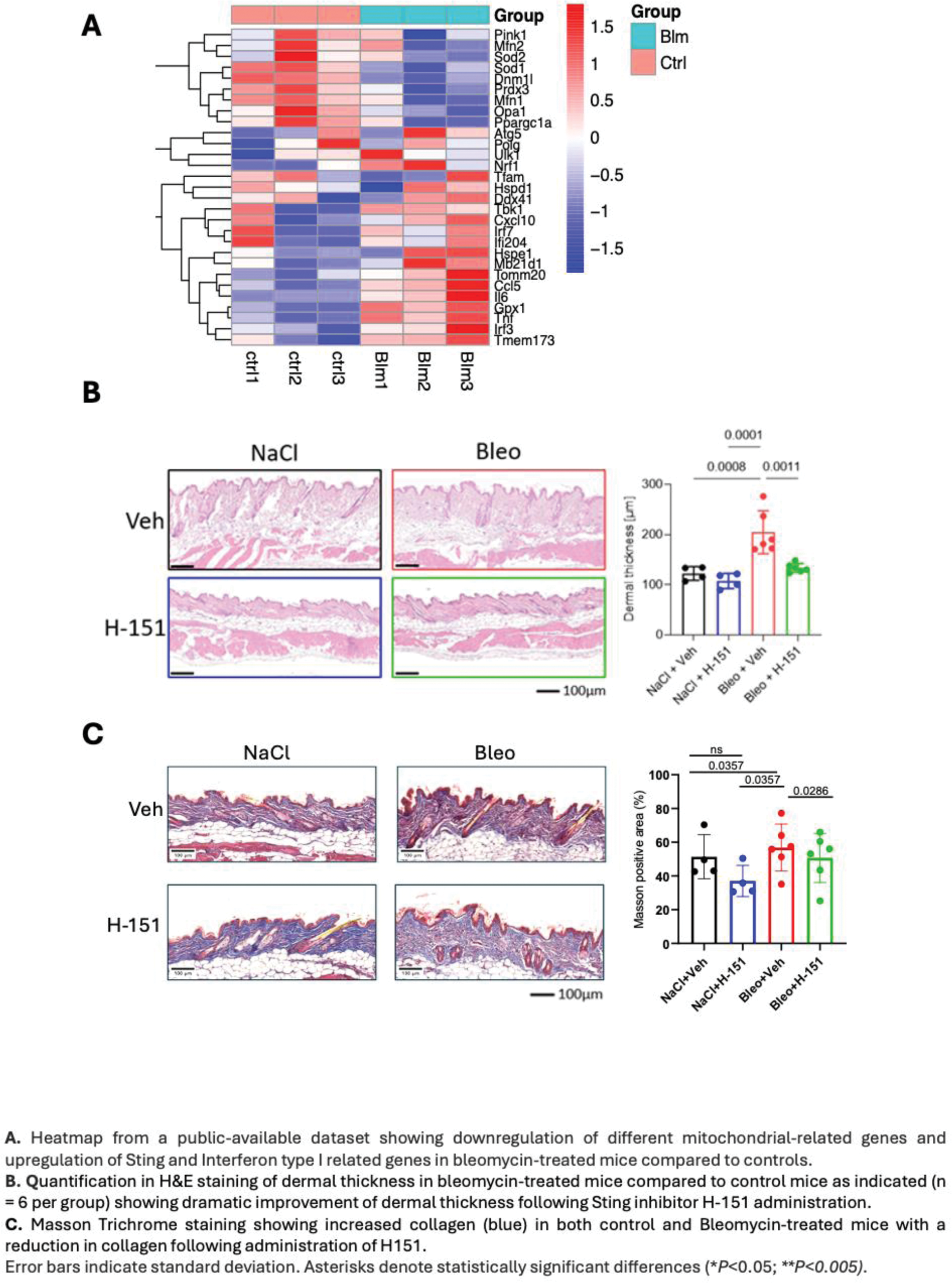
Methods: RNA-seq Analysis: RNA-seq data (dataset GSE231692) from the FaSScinate clinical trial were examined to investigate the expression of genes related to mitochondria, cGAS-STING signaling, and interferon pathways. Skin Biopsies: Skin biopsies from SSc patients and healthy controls (HCs; n = 10 each) were analyzed by RT-PCR and immunohistochemistry (IHC) to examine cGAS-STING pathway components (cGAS, STING, IRF3, and type I interferons). Mitochondrial Dysfunction: Assessed by quantifying TFAM and MTND1 gene expression. Fibroblasts isolated from skin biopsies (SSc vs. HCs) underwent Seahorse analysis and ATP assays to evaluate glycolysis and mitochondrial respiration. Mitochondrial Stress and STING Activation: Fibroblasts were treated with a mitochondrial uncoupler FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), followed by confocal microscopy to track STING nuclear translocation; IFNB gene induction was evaluated by RT-PCR. Bleomycin-Induced Fibrosis (BIF): Public datasets were analyzed to compare mitochondrial and STING-related gene expression in BIF mice versus controls. Finally, BIF mice received intraperitoneal H-151, a selective STING inhibitor, to assess the therapeutic potential of STING inhibition.
Results: The expression of genes associated to mitochondrial homeostasis, Interferon and cGAS-sting pathways are perturbed in the skin of SSc vs HCs. Specifically, compared to HCs, SSc skin overexpress STING1, cGAS, TBK1, IFI16, and STAT1 (Figure 1). Overexpression of STING, cGAS, and IFNB1 was further confirmed in rtPCR (p < 0.01). Consistently, SSc skin samples displayed robust activation of the cGAS-STING pathway demonstrated by elevated levels of cGAS, STING, and pIRF3 proteins in IHC. This pointed toward a mitochondrial dysfunction-induced cGAS-STING-IFN activation in skin of SSc. Mitochondrial dysfunction emerged as a prominent feature in SSc skin, characterized by significantly reduced TFAM expression (p < 0.01) and a trend toward lower MTND1 levels. In line with gene expression data, metabolic profiling revealed that SSc fibroblasts exhibited impaired mitochondrial respiration, including diminished spare respiratory capacity (p < 0.05) and decreased ATP production, along with increased basal oxygen consumption (p < 0.05) and a greater reliance on glycolysis compared to HCs. Interestingly, FCCP-induced mitochondrial stress significantly enhanced STING nuclear translocation that was prominent in SSc fibroblasts compared to HCs, inducing IFNβ gene expression and demonstrating a direct link between mitochondrial dysfunction, STING activation, and IFNβ production. Bleomycin-treated mouse models mirrored these findings, exhibiting compromised antioxidant defenses (reduced Sod1 and Prdx3 ) and upregulation of STING-related genes ( Irf3 , Irf7 , Tmem173 ). Pharmacological inhibition of Sting using H-151 in vivo significantly reduced dermal thickness and collagen deposition in bleomycin-induced skin fibrosis (BIF) mice (Figure 2), underscoring the role of Sting in fibrosis and the possible therapeutic potential of targeting the STING pathway in SSc.
Conclusion: Mitochondrial dysfunction is a driver of cGAS-STING pathway activation and subsequent IFN-I production in SSc. The potent anti-fibrotic effects of STING inhibition in experimental models suggest a promising therapeutic avenue for addressing the unmet needs of SSc patients.
REFERENCES: [1] Zhou X, Trinh-Minh T, Tran-Manh C, Gießl A, Bergmann C, Györfi AH, Schett G, Distler JHW. Impaired Mitochondrial Transcription Factor A Expression Promotes Mitochondrial Damage to Drive Fibroblast Activation and Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2022 May;74(5):871-881.
[2] Wobma H, Shin DS, Chou J, Dedeoğlu F. Dysregulation of the cGAS-STING Pathway in Monogenic Autoinflammation and Lupus. Front Immunol. 2022 May 27;13:905109.
[3] Kim, J, Kim, HS. & Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp Mol Med 55 , 510–519 (2023).
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (