

Background: Despite the outburst of knowledge on the pathogenesis of idiopathic inflammatory myopathies (IIM), the immune mechanisms underlying those disease are still not fully understood. The spectrum of IIM symptoms extends far beyond the muscles and interstitial lung disease is one of the most common complications. Preceding infections of the respiratory tract and exposure to inhaled particles were demonstrated to increase the risk of IIM in several studies [1, 2]. Seasonality of the IIM onset supports the idea of pathogens as plausible trigger factors [3]. Therefore, airway epithelium emerges as a promising milieu for the initiation of autoimmunity in IIM.
Objectives: We aimed to assess the immune responses within nasal airway epithelium of patients with IIM in response to viral triggers and to compare them between IIM and healthy individuals as well as between IIM subtypes.
Methods: Nasal epithelial cells (HNEC), obtained from 11 patients with IIM and 9 healthy controls (HC), were harvested by gentle passage of the middle meatus with cytology brushes, isolated and cultured on collagen-coated plates. Supernatants of HNEC were used as probes after 24-hour incubation with and without 1 μg/ml Poly(I:C) LyoVec™, a synthetic dsRNA analogue, imitating viral infection, and analysed using the Flexpanel assay (Olink Proteomics, Sweden). Protein levels were reported as Normalized Protein Expression (NPX) values on a log2 scale after normalization against internal controls. The NPX values of the studied proteins in the supernatants of both unstimulated cells and cells stimulated with Poly(I:C) for 24 h, as well as ΔNPX (ΔNPX = NPX poly(I:C) – NPX w/o tretatment ) were compared between the IIM (including also each of the IIM subtypes) and HC groups. Statistical analysis was performed with U-Mann Whitney and Wilcoxon tests.
Results: Among 11 patients with IIM, 4 were diagnosed with dermatomyositis (DM), 3 with antisynthetase syndrome (AS), 3 with immune-mediated necrotizing myopathy (IMNM) and 1 with polymyositis (PM). After incubation with Poly-IC noticeable releases of IFNB1, TNF, IL6, INFNA2, IL33, TSLP, IL18, TGFB1, PTX3, INFL1, CCL3, IL1A, CSF2, IL17C, CXCL10, IL1B were demonstrated in both IIM and HC groups. Patients with IIM and HC did not differ as for the values of assessed markers in both unstimulated and stimulated cells’ supernatants, yet ΔCCL11 was statistically lower in IIM subgroup. Comparing HC and individual subsets of IIM, in the supernatants of unstimulated cells we noted higher median values of IFNA2 and lower median values of IFNL1 in the AS group, lower median values of TNF in DM patients and higher median values of IL17C in IMNM group. After incubation with Poly(I:C)LyoVec™, in the supernatants of patients with AS median values of CCL3 were higher as compared to HC. Prominent release of INFL1, TSLP and IL17C was found upon stimulation in AS group as compared to HC (Figure 1). Comparing patients with AS to other IIM subtypes, we found statistically lower median values of IFNL1 in non-stimulated cells with higher ΔINFNL1 as well as higher median values of PTX3 and CCL3 in the supernatants of incubated cells. IFNB1 showed notable upregulation in the AS group, while it was downregulated in remaining subgroups. Figure 1 presents the changes in protein expression in IIM and HC after Poly(I:C)LyoVec™ stimulation.
Changes in protein expression (ΔNPX) after treatment with Poly(I:C)LyoVec™ in patients (IIM DM, IMNM, ASS) and healthy controls (HC). Data are presented as the median ΔNPX±range, with the range shown as error bars.
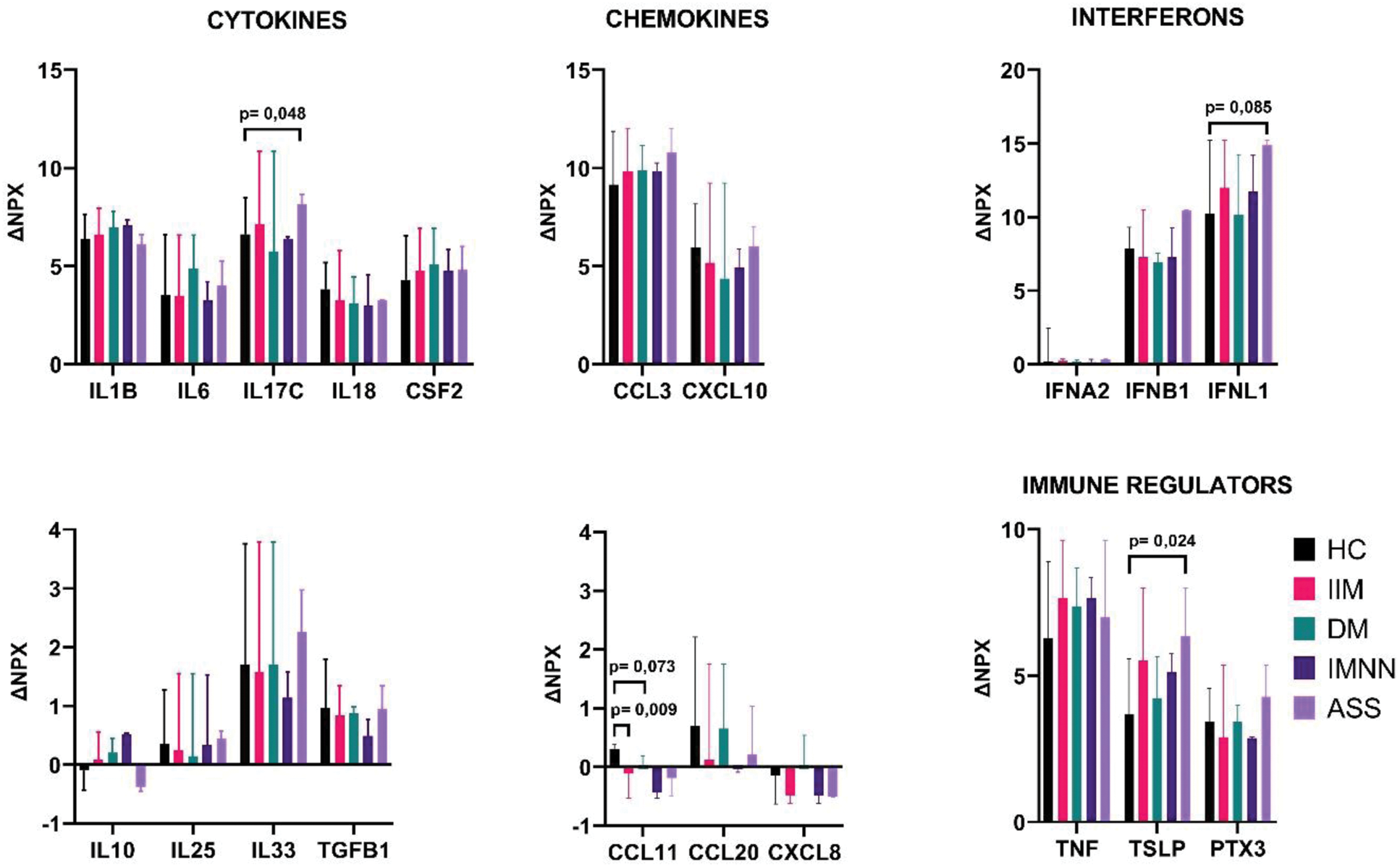
Conclusion: Immune response to viral infection in airway epithelium does not differ in patients with IIM as a whole as compared to HC, yet significant differences are evident for individual IIM subtypes. Unique pattern is observed mostly for AS subtype, which supports the potential involvement of respiratory infections in the pathogenesis of antisynthetase syndrome.
REFERENCES: [1] Svensson J, Holmqvist M, Lundberg IE, Arkema E V. Infections and respiratory tract disease as risk factors for idiopathic inflammatory myopathies: A population-based case-control study. Ann Rheum Dis 2017;76:1803–8.
[2] Costa AN, Kawano-Dourado L, Shinjo SK, Carvalho CRR, Kairalla RA. Environmental exposure in inflammatory myositis. Clin Rheumatol 2014;33:1689–90.
[3] Sarkar K, Weinberg CR, Oddis C V, Medsger TA, Plotz PH, Reveille JD, et al. Seasonal influence on the onset of idiopathic inflammatory myopathies in serologically defined groups. Arthritis Rheum 2005;52:2433–8.
Acknowledgements: National Science Centre Poland, grant number 2020/37/N/NZ6/01442.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (