

Background: The poor prognosis of systemic sclerosis (SSc) underscores the urgent need to prevent onset of established disease. Understanding the molecular events that drive the transition from pre-fibrotic pre-stages of the diseases such as very early SSc (veSSc) to established fibrotic SSc meeting classification criteria is essential for developing effective treatments.
Objectives: We aimed to identify key cellular and molecular alterations, characterizing the very early stages of SSc before clinically apparent fibrosis occurs.
Methods: We recruited patients with veSSc (n=6) without skin or other organ fibrosis, early established fibrotic SSc (eSSc, n=7), and healthy controls (HC, n=6). veSSc was defined according to the VEDOSS criteria described previously [1], while fibrotic eSSc patients met the 2013 ACR/EULAR criteria with disease duration <5 years since their first non-Raynaud manifestation. HC participants were matched by age, sex, and ethnicity. Site-matched forearm skin biopsies were collected following the Chromium Next GEM protocol. Raw reads were aligned to the human genome with Cellranger and processed using Seurat. Sample quality control included CellBender for ambient RNA, feature thresholding for low-quality cells, and DoubletFinder for doublet removal. Batch effects were addressed using Harmony integration. Cell types were manually annotated with the top 25 highly expressed genes at optimized cluster resolution based on published skin datasets. Compositional analysis to account for cell-type variations among three groups was performed using Speckle. Differential gene expression analysis (DGEA) was performed pairwise on a mixed model (MAST; |log2FC|>1, adjusted p-value<0.05). Pathway analyses of differentially expressed genes (DEGs) were conducted using clusterProfiler with the Gene Ontology (GO) database. Cell-cell communication analysis identified differentially upregulated ligand-receptor pairs across cell types in veSSc vs. HC using MultiNicheNet. With an interest in fibroblasts, we utilized STACAS integration to refine the fibroblast subclusters. Pathway analyses of highly expressed marker genes and DEGs were conducted respectively to characterize the identified subclusters relevant to disease pathology.
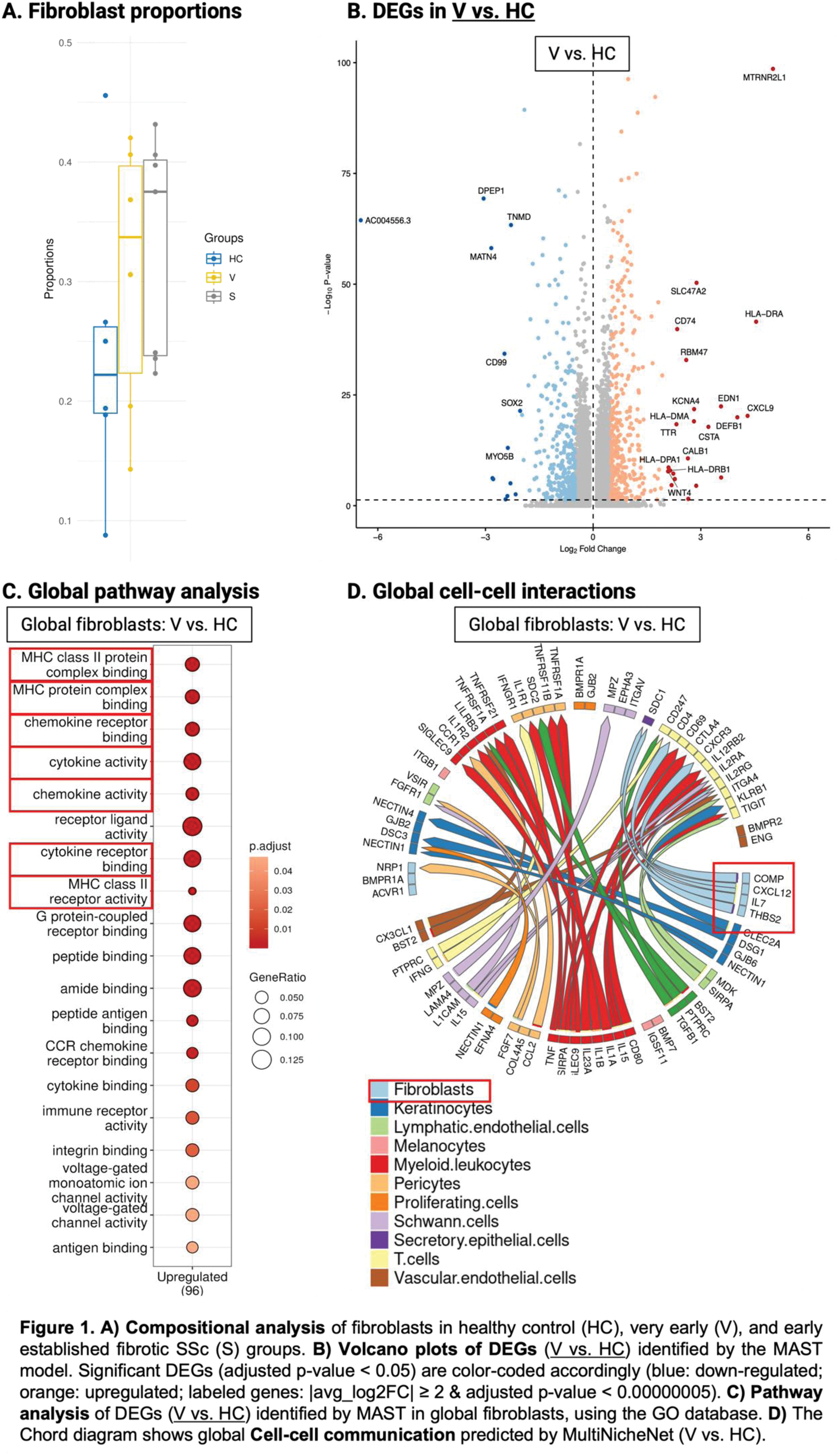
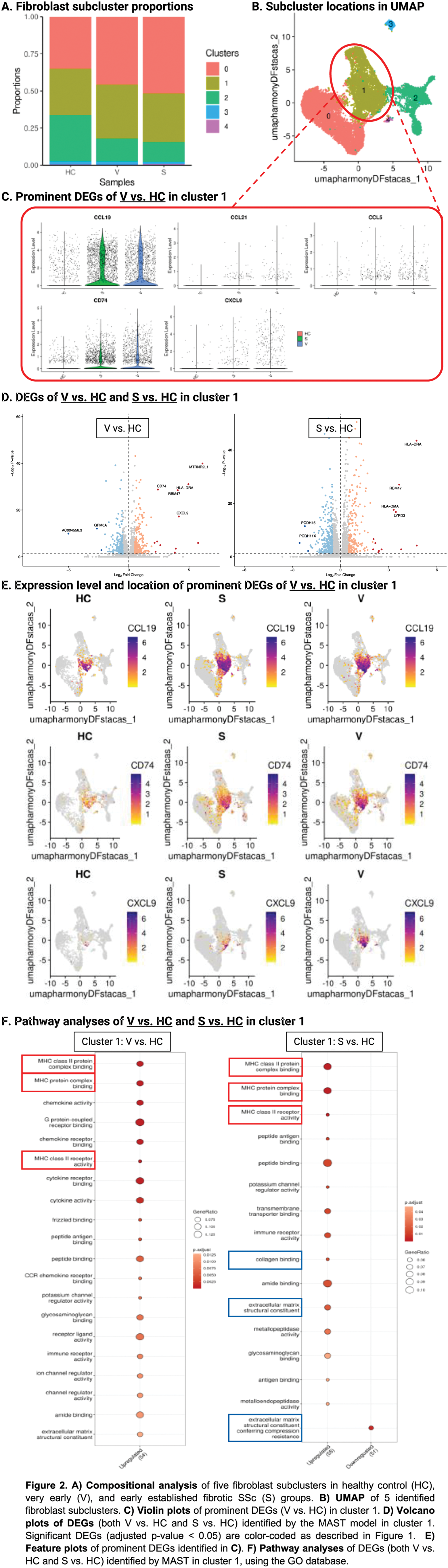
Results: We identified 61,743 cells corresponding to 13 cell types, including 18,755 fibroblasts (veSSc=6,322; eSSc=8,831; HC=3,602). Cell compositional analysis showed increased fibroblast abundance in both veSSc (proportional ratio: 1.28) and eSSc patients as compared to HC (Figure 1A). When comparing veSSc to HC, pairwise DGEA in fibroblasts resulted in 195 DEGs using MAST. Notably, veSSc was characterized by a pro-inflammatory phenotype in fibroblasts, while a pro-fibrotic phenotype was not found and only occurred in eSSc, the pro-inflammatory fibroblast phenotype was maintained in eSSc. Specifically, in veSSc, HLA class II genes encoding for histocompatibility antigen chains (HLA-DRA, HLA-DPA1, and HLA-DRB1) and pro-inflammatory indicator genes ( CXCL9 : T-cell activating chemokine, CD74 : surface receptor for the pro-inflammatory macrophage migration inhibitory factor) were among the top upregulated genes compared to HC (Figure 1B). In addition, pathway analyses showed that the DEGs in veSSc fibroblasts were strongly associated with upregulated MHC class II, cytokine and chemokine activity, such as “MHC class II protein complex binding” and “MHC class II receptor activity”, “cytokine activity”, and “chemokine activity” (Figure 1C). Cell-cell communication analysis revealed a predicted enhanced ligand-receptor signaling from fibroblasts towards T-cells in veSSc, in particular between the fibroblast-derived ligand CXCL12 and CD4 and CXCR3 on T-cells, as well as between IL7 from fibroblasts and IL2RG on T-cells. These findings indicate the presence of pro-inflammatory crosstalk in patients at risk for developing established fibrotic SSc (Figure 1D). In our dataset, we identified five fibroblast subclusters, among which cluster 0 (veSSc=2,892; eSSc=4,570; HC=1,260) and cluster 1 (veSSc=2,290; eSSc=2,881; HC=1,119) were the most abundant (Figure 2A). We identified cluster 1 as a pro-inflammatory fibroblast subcluster (expressing CCL19 , etc) (Figure 2B-C). In veSSc vs. HC, this cluster yielded 87 DEGs in MAST, with overlapping genes consistent with those reported in the global fibroblast DEGs analysis (Figure 2D-E). Pathway analyses of cluster 1 in veSSc revealed upregulation of the pro-inflammatory pathways observed in the global fibroblast analysis, suggesting that these pathways in the global population were predominantly driven by cluster 1 (Figure 2F). Moreover, the pro-inflammatory characteristics of cluster 1 were consistently observed in both DEGA and ORA analyses when comparing eSSc to HC, suggesting that pro-inflammatory state in fibroblasts is a common mechanism underlying early stages of systemic sclerosis.
Conclusion: Our findings reveal distinct cellular and molecular alterations in veSSc. Specifically, we identified a prominent pro-inflammatory fibroblast signature in veSSc which could be assigned to one fibroblast subcluster. These changes precede clinically evident fibrosis and are maintained in fibrotic eSSc. The identification of pro-inflammatory pathways, such as MHC class II activation in fibroblasts and fibroblast-T-cell interactions, highlights the potential for targeted anti-inflammatory interventions to prevent fibrotic disease onset. These findings provide critical insights into the very early pathogenesis of SSc and inform clinical trials to prevent disease.
REFERENCES: [1] Muraru et al. (2024). “Arthritis in patients with very early systemic sclerosis: a comprehensive clinical and prognostic analysis.” Rheumatology.
Acknowledgements: NIL.
Disclosure of Interests: Lumeng Li: None declared , Elena Pachera: None declared , Rucsandra Dobrota Actelion, advisory board for Boehringer-Ingelheim, Grants/research support from Pfizer, Actelion, Iten-Kohaut Foundation, and Walter und Gertrud Siegenthaler Fellowship. Congress participation support from Amgen, Otsuka, Sinziana Muraru Congress participation support from AstraZeneca, Kristina Buerki: None declared , Carina Mihai Speaker fees from MED Talks Switzerland, Mepha, MedTrix, Novartis, and PlayToKnow, Consultancy relationship with Boehringer Ingelheim and Janssen, Congress support from Boehringer Ingelheim in the last three calendar years, Muriel Elhai Boehringer Ingelheim, Grant/research support from Pfizer, Novartis Foundation for Bio-Medical Research, Iten Kohaut Foundation, Kurt und Senta Herrmann Foundation, Foundation for Research in Rheumatology (FOREUM), Walter and Gertrud Siegenthaler Foundation, Theodor und Ida Herzog-Egli – Stiftung and Association des Sclérodermiques de France (ASF). Congress Support from Astrazeneca and Janssen, Laura Much: None declared , Astrid Hofman: None declared , Pietro Bearzi: None declared , Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, AbbVie, ARXX, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant Therapeutics, Roche and Werfen, Boehringer Ingelheim, Janssen, Oliver Distler OD has/had consultancy relationships with and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, OD has/had consultancy relationships with and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, BI, Kymera, Mitsubishi Tanabe, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (