

Background: The pathogenesis of synovitis in systemic sclerosis (SSc) remains poorly understood. In the lack of evidence-based therapies, SSc patients with synovitis are commonly treated with regimens used for rheumatoid arthritis (RA), though their effectiveness is unknown. Previously, we identified a pauci-immune histopathology in SSc synovium and a prominent interferon (IFN) signature in synovial fibroblasts (SF), contrasting strong tumour necrosis factor (TNF) signalling in RA SF [1].
Objectives: To further investigate the characteristics of SSc synovitis in comparison to RA and non-inflammatory control (NIC) synovium, with a focus on SF and myeloid cells.
Methods: Histological classification was performed on synovium from 11 SSc, and 7 RA patients with active synovitis and compared to synovium from 6 NIC. Tissue samples from 6 SSc, 6 RA, and 6 NIC were dissociated for single-cell RNA sequencing (scRNA-seq), yielding 78,128 total cells (SSc: 21,248; RA: 32,062; NIC: 24,818) analysed with Seurat (v4.3). Re-clustering and integration (STACAS) focused on 14,328 SF (SSc: 4,572; RA: 5,847; NIC: 3,909) and 18,972 myeloid cells (SSc: 5,602; RA: 8,105; NIC: 5,265), manually annotated based on established literature [2, 3]. Differential abundance, pairwise differential gene expression (MAST), pathway (clusterProfiler) and transcription factor (TF) activity analyses (decoupleR), and gene module score calculations were performed. Protein levels were determined using ELISA on supernatants from an in vitro 3D SF micromass model after long-term stimulation (14 days) with TNF, IFN-α and IFN-γ.
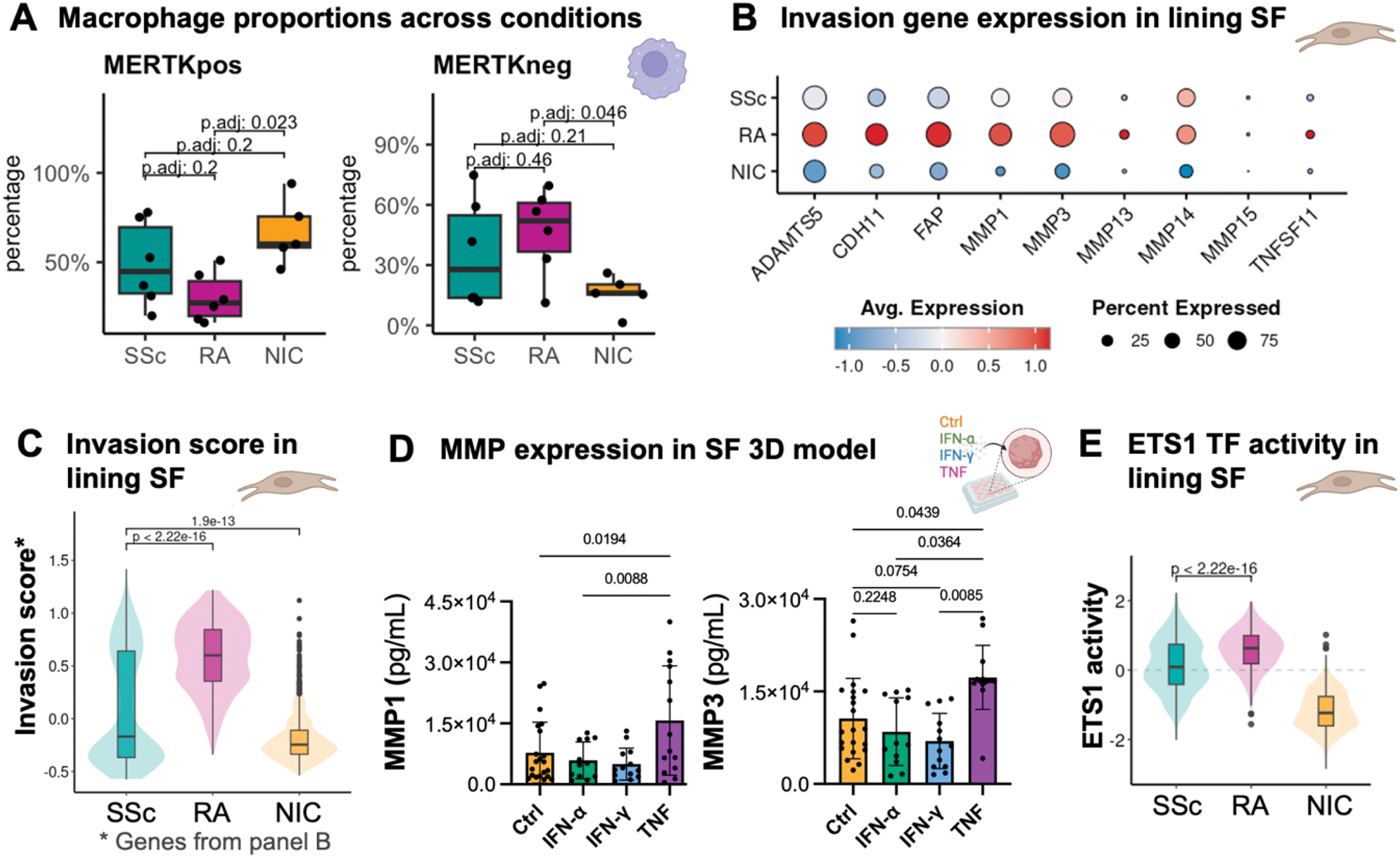
Results: Among SSc patients, 27% were male, with a median age of 65 years (interquartile range (IQR) 59.5–71.5) and a disease duration of 2.5 years (IQR 0.6–7.0). Most had limited cutaneous disease (91%), and 7/11 were treatment-naïve. RA patients were predominantly female (83%), with a median age of 57.5 years (IQR 54.5–63.5) and a disease duration of 7.0 years (IQR 5.4–15.4). On a histological level, acute inflammation was more frequently observed in RA as compared to SSc synovitis (86% vs. 9%, p<0.01). At the single-cell level, in the myeloid compartment, NIC samples were predominantly composed of MERTK pos tissue-resident macrophages, whereas RA and SSc exhibited infiltration by MERTK neg macrophages (Figure 1A). In RA, these MERTK neg macrophages expressed higher levels of SPP1 (osteopontin) (log 2 FC=-3.13, padj=1.2e-115 compared to SSc), a marker of inflammation and bone remodelling, and were enriched in TNF signalling. In contrast, MERTK neg macrophages in SSc displayed less inflammatory signalling, with practically absent SPP1 expression and stronger enrichment in the IFN pathways, consistent with our previous findings in SSc SF [1]. It is well established that in RA, the lining layer becomes invasive, contributing to joint destruction, while inflammation is occurring in the sublining layer. When comparing these two compartments across SSc, RA and NIC, we observed the highest number of differentially expressed genes in the lining SF (SSc: n=152 vs. RA: n=164, for |log 2 FC|>0.25 & padj<0.01,). Notably, several invasion markers, including MMP1 (log 2 FC=-0.933, padj=5.57e-11) and MMP3 (log 2 FC=-0.846, padj=5.27e-27), were significantly upregulated in RA lining SF compared to SSc and were nearly absent in NIC (Figure 1B invasion marker genes, Figure 1C module score). Supporting our hypothesis, MMP1 and MMP3 expression increased following TNF stimulation relative to IFN-α and IFN-γ in a 3D SF micromass model (Figure 1D). Consistently, ETS1 (Figure 1E), a transcription factor (TF) critical for joint erosions [4], was highly active in RA lining SF, but significantly less in SSc.
Conclusion: Taken together, these results point towards a less inflammatory and less invasive synovium in SSc compared to RA, which might explain a lower potential for erosive joint damage. The pronounced IFN signature detected in both SF and macrophages further supports the hypothesis that IFN pathways could serve as potential therapeutic targets in SSc synovitis.
REFERENCES: [1] Geiss, Celina, et. al. Ann Rheum Dis (2024).
[2] Zhang, Fan, et al. Nature (2023).
[3] MacDonald, Lucy, et al. Immunity (2024).
[4] Yan, Minglu, et al. Nat Immunol (2022).
Abbreviations: RA = rheumatoid arthritis, SF = synovial fibroblasts, SSc = systemic sclerosis, TF = transcription factor. Values are indicated as median and interquartile range.
Acknowledgements: This project is funded by FOREUM – Foundation for Research in Rheumatology. We thank our patient research partners Joëlle Messmer & Andreas Eisenring.
Disclosure of Interests: Celina Geiss: None declared , Miranda Houtman: None declared , Raphael Micheroli: None declared , Camino Calvo Cebrian: None declared , Alexandra Khmelevskaya: None declared , Kristina Buerki: None declared , Oliver Distler 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur, UCB, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), Co-founder of CITUS AG, 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur, UCB, BI, Kymera, Mitsubishi Tanabe, UCB, Caroline Ospelt: None declared , Muriel Elhai Boehringer Ingelheim, Pfizer, Novartis Foundation for Bio-Medical Research.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (