

Background: Delays in diagnosis of psoriatic arthritis (PsA) are associated with indolent and non-specific signs and symptoms, and often result in physical impairment and poorer global health outcomes in the long term [1, 2, 3, 4]. Digital tools such as machine learning algorithms have the potential to address diagnostic delays by identifying patients earlier in their disease course [5].
Objectives: The purpose of this study was to develop and evaluate a novel machine learning model for identifying patients at risk of having undiagnosed PsA among patients who saw primary care services at Mayo Clinic.
Methods: Patients receiving primary care services (n=395,918 patients) at Mayo Clinic between 2012 and 2022 were split into training and testing sets. Cases with incident PsA (n=326) and controls with no evidence of PsA (n=395,592) were identified in both sets. For model training, each patient was assigned a prediction date, which preceded the initial diagnosis of PsA for cases, and was randomly assigned to controls. A gradient boosted trees algorithm was trained using electronic medical record (EMR) data documented during the two years prior to each patient’s prediction date. Input features included age, sex, diagnosis codes, medication prescriptions and laboratory results. The model was then evaluated in the test set on predictions made on 1 January 2018 with input data from the preceding two years. Area under the curve (AUC) was used to assess the model’s ability to discriminate between new cases of PsA diagnosed during and after 2018 and controls.
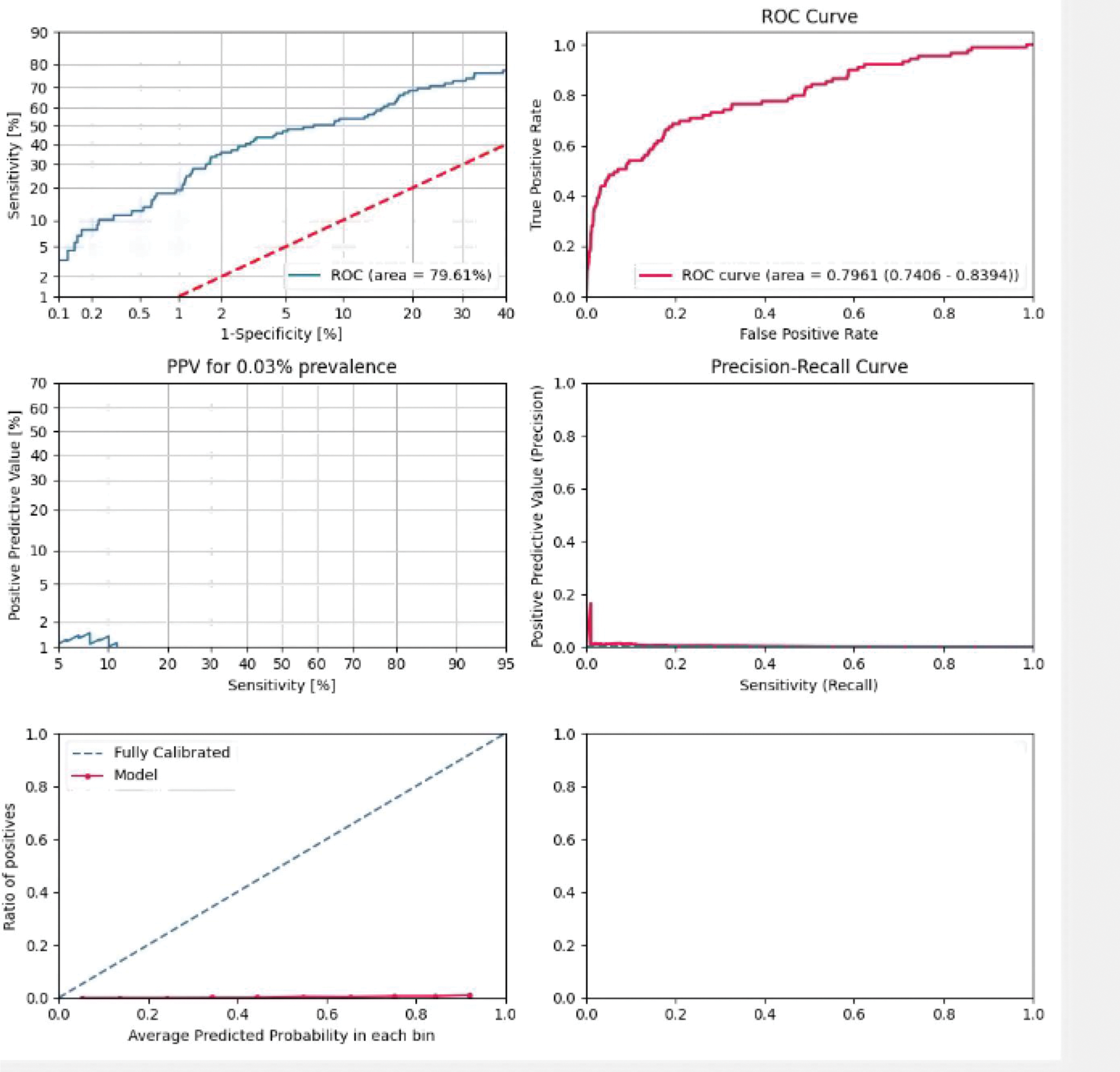
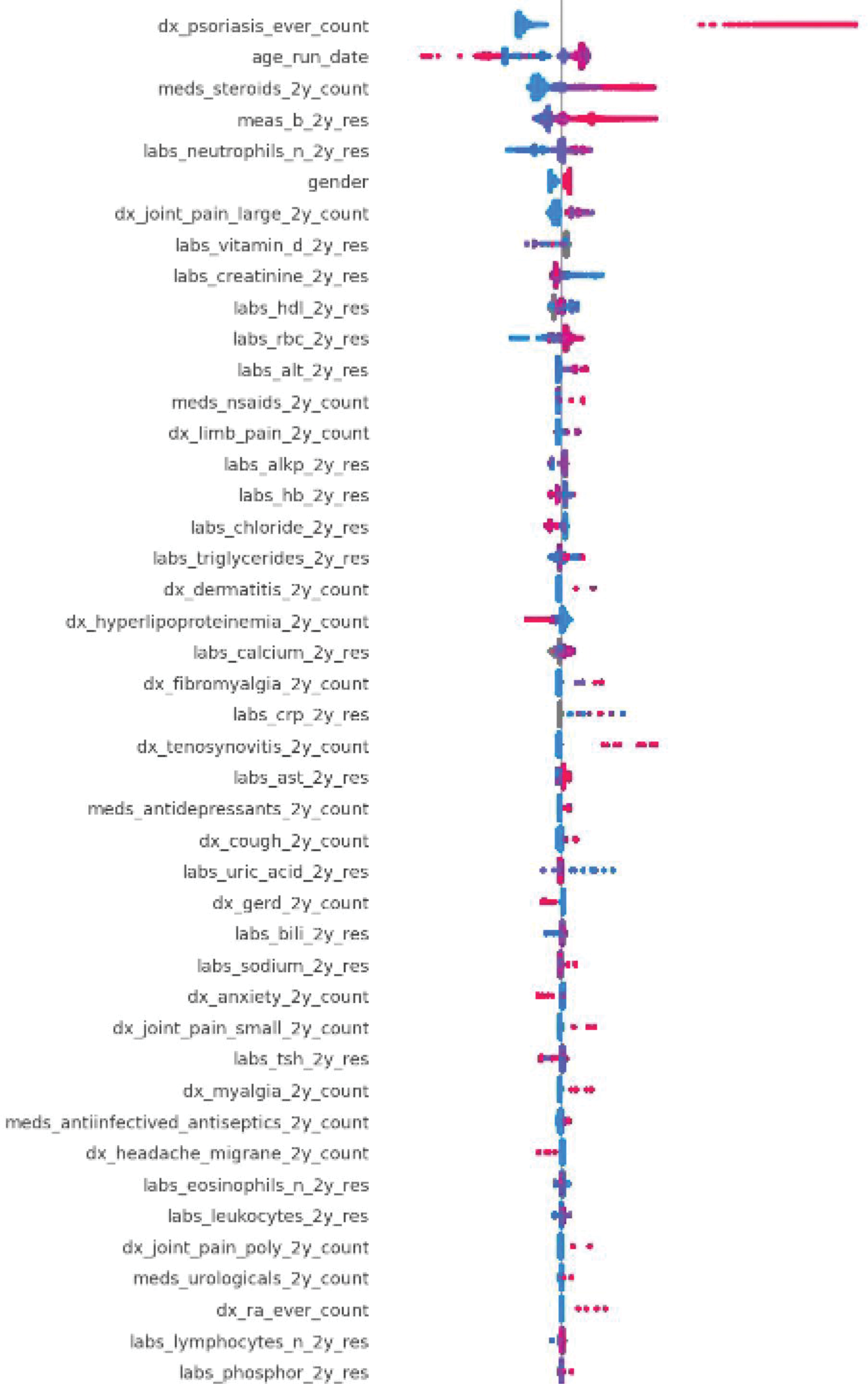
Results: The testing set included 89 patients with PsA (60 female; mean age 54.2, standard deviation [SD] 14.0) and 284,480 control patients (167,018 females; mean age 51.9, SD 18.2). The AUC on the test was 79.6% (Figure 1). The top predictive features preceding the initial PsA diagnosis include diagnoses of psoriasis, joint pain and tenosynovitis, glucocorticoid and NSAID use, high body mass index, elevated CRP, ALT, triglycerides and decreased HDL (Figure 2).
Conclusion: The model displayed good performance in its ability to discriminate between cases of PsA and controls. The model selected input features associated with preexisting psoriasis, joint inflammation and an adverse metabolic profile [6]. Implementation of the model may help identify patients with undiagnosed PsA in the primary care population using EMR. Improving time to diagnosis could help patients receive treatment and reduce downstream sequelae from untreated disease.
REFERENCES: [1] Sørensen J, Hetland ML; all departments of rheumatology in Denmark. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis . 2015;74(3):e12. doi:10.1136/annrheumdis-2013-204867
[2] Kiliç G, Kiliç E, Tekeoğlu İ, et al. Diagnostic delay in psoriatic arthritis: insights from a nationwide multicenter study. Rheumatol Int . Published online October 8, 2023. doi:10.1007/s00296-023-05479-z.
[3] Tillett W, Jadon D, Shaddick G, et al. Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis . 2013;72(8):1358-1361. doi:10.1136/annrheumdis-2012-202608
[4] Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis . 2015;74(6):1045-1050. doi:10.1136/annrheumdis-2013-204858
[5] Fagni F, Knitza J, Krusche M, Kleyer A, Tascilar K, Simon D. Digital Approaches for a Reliable Early Diagnosis of Psoriatic Arthritis. Front Med (Lausanne ). 2021;8:718922. Published 2021 Aug 11. doi:10.3389/fmed.2021.718922
[6] Aljohani R. Metabolic Syndrome and Its Components in Psoriatic Arthritis. Open Access Rheumatol . 2022;14:7-16. Published 2022 Feb 17. doi:10.2147/OARRR.S347797
Receiver operating characteristics curve displaying the discriminative ability of the model over all thresholds.
SHAP plot indicating the top features contributing to model classification output. Features that are derived from diagnosis codes (‘dx’), lab results, prescriptions are indicated. Female is coded as 1 for gender.
Why press conference: We believe this research represents a development of new digital tools that can help identify patients that should be seen by rheumatologists sooner, which will allow for faster diagnosis and treatment of disease.
Acknowledgements: NIL.
Disclosure of Interests: Michael Dreyfuss Medical Director at Predicta Med with options. Current Medical Director at Predicta Med with options, Dan Riesel Data Scientist at Predicta Med with options, Data Scientist at Predicta Med, Guy Shalev Data Scientist at Predicta Med with options, Data Scientist at Predicta Med, Douglas White Advisory board at Predicta Med with options, Or Ramni Data Scientist at Predicta Med with options, Data Scientist at Predicta Med, Benjamin Getz CTO at Predicta Med with options, CTO at Predicta Med, Daniel Underberger CMO at Predicta Med with options, CMO at Predicta Med with options, Shlomit Steinberg-Koch CEO at Predicta Med with options, CEO at Predicta Med, Elena Myasoedova: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (