

Background: Systemic lupus erythematosus (SLE) is a heterogenous disease for which there are few licensed therapies. In clinical trials, response to treatment is usually assessed a single ‘landmark’ time point, despite SLE typically running a relapsing-remitting course.
Objectives: In a real-world cohort, i) to uncover trajectories of clinical response to rituximab, taking initial disease severity and longitudinal course into account, ii) to determine if baseline clinical features distinguish such trajectories and iii) to compare these trajectories to landmark measures of clinical response.
Methods: Participants recruited to the UK prospective observational study, The British Isles Lupus Assessment Group Biologics Register (BILAG-BR) between July 2010-November 2023 were included if treated with rituximab. Trajectories of the numerical BILAG-2004 score (nBILAG) over a one-year period were uncovered using group-based latent class trajectory models (LCTM) to identify clusters of individuals who followed a similar pattern of disease activity after rituximab treatment. Optimal models were selected based on model fit and clinical relevance. Baseline demographics, clinical features and serology across the groups were compared using multivariate logistic regression models. Binary clinical response was assessed using a modified version of the British Isles Lupus Assessment Group–Based Composite Lupus Assessment (mBICLA) which excluded physician global assessment (PGA). Multiple imputation with chained equations was used to handle missing mBICLA data in Stata V14.3. Comparisons between trajectory assignment and mBICLA response were completed descriptively.
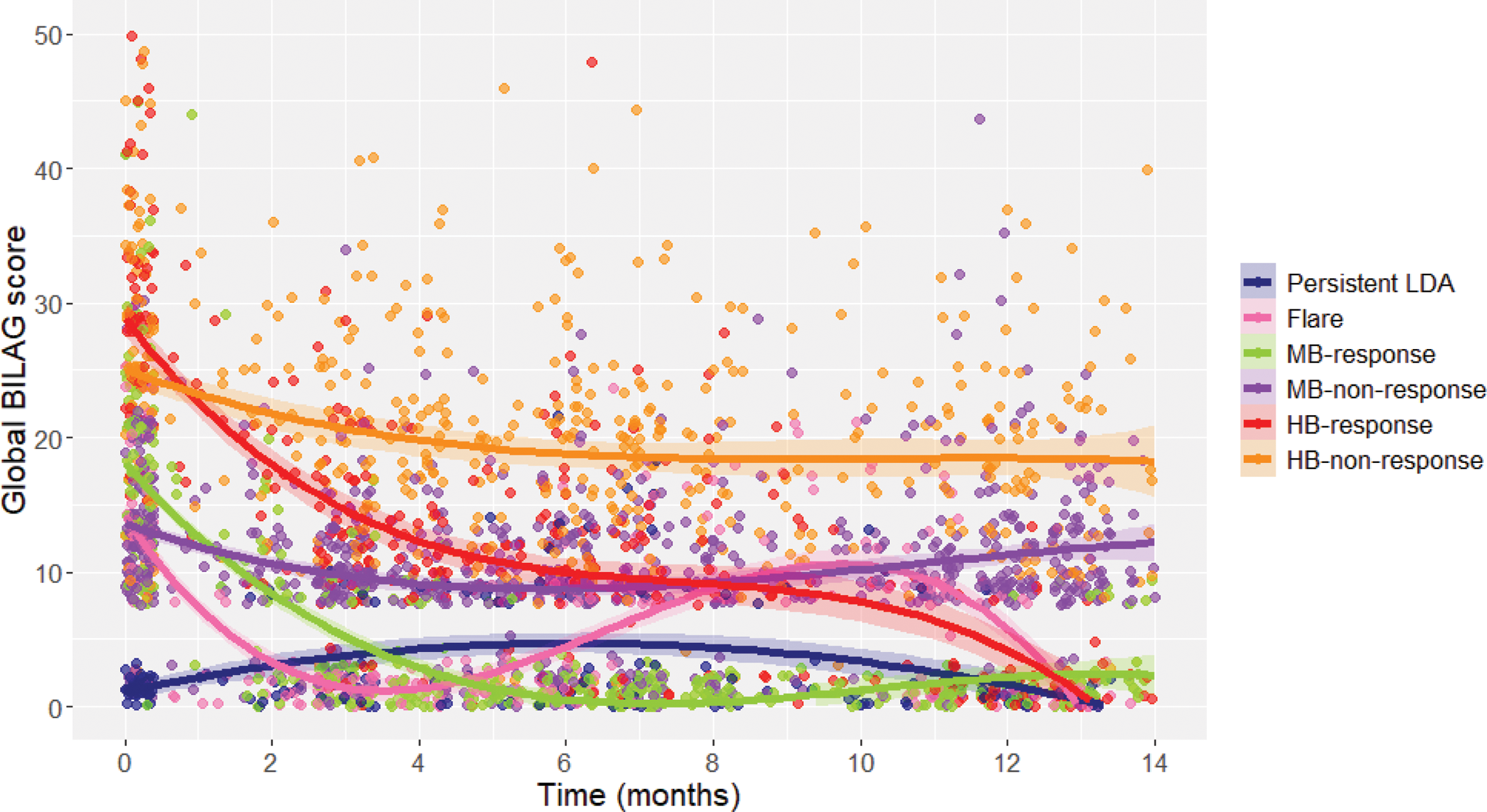
Results: Of 1292 individuals given rituximab treatment, 151 without a nBILAG score recorded were excluded and 1141 were included in the analysis. We identified six longitudinal clusters – high-baseline disease activity responders (12%), high-baseline disease activity non-responders (19%), moderate-baseline disease activity responders (21%), moderate-baseline disease activity non-responders (31%), responder-flare (9%) and persistent low disease activity (8%) (Figure 1). Using a landmark assessment across the cohort, mBICLA was met by 55% (95% CI 51-59) at six months and 59% (95% CI 55-63) at twelve months. Disease activity in the responder-flare cluster increased around nine months post-rituximab, so whilst 67% would been classed as attaining the mBICLA response at 6 months, a high proportion would have been excluded due to a flare prior to the 12-month landmark. The responder-flare trajectory was associated with active musculoskeletal disease at baseline (odds ratio (OR) 1.70 [interquartile range (IQR) 1.30-2.81] compared with the moderate-baseline disease activity responder and non-responder classes. High-baseline disease activity response trajectory was associated with higher baseline nBILAG (OR 1.05 [IQR 1.03-1.08] per unit increase), anti-dsDNA positivity (OR 1.81 [IQR 1.03-3.18]) and anti-RNP negativity (OR 0.58 [IQR 0.34-0.99]) compared with high-baseline disease activity non-responders.
Conclusion: We identified six distinct trajectories of response to rituximab that better reflect longitudinal disease activity than binary assessment of clinical response at an arbitrary timepoint. Non-response with high disease at baseline is associated with anti-RNP positivity and anti-dsDNA negativity. The response-flare trajectory was associated with active musculoskeletal involvement. Such trajectory groups may help better accommodate the variability over time in SLE patients, particularly in those with active arthritis.
REFERENCES: NIL.
The six class latent trajectory model of disease activity after rituximab treatment. Abbreviations: BILAG, British Isles Lupus Assessment Group; LDA, low disease activity; MB, moderate baseline disease activity; HB, high baseline disease activity.
Acknowledgements: NIL.
Disclosure of Interests: Mia Rodziewicz MR is Medical Research Council Clinical Training Fellow based at the University of Manchester supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (award reference MR/N025989/1), Roche Pharma, Eli Lilly and Company Limited, UCB Pharma, Novartis, the University of Liverpool and the University of Manchester. Stephanie Shoop-Worrall: None declared. Sarah Dyball: None declared. Emily Sutton: None declared. John Reynolds Otsuka and Merck Sharp & Dohme, Ben Parker: None declared. Ian Bruce Astra Zeneca, Janssen, GSK, Novartis, Astra Zeneca, Janssen, GSK, Novartis, Takeda, BMS, Grants to Institution from Astra Zeneca, Janssen, GSK, Otsuka.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (