

Background: Patients with systemic lupus erythematosus (SLE) have a ≥ 2- to 3-fold increased risk of cardiovascular (CV) disease, including myocardial infarction, congestive heart failure, cerebrovascular disease, and overall CV disease–related mortality compared with the general population [1,2]. The mechanisms underlying the increased risk of CV disease in patients with SLE are complex and further complicated by immune system dysregulation, CV comorbidities, and inflammation. Janus kinase (JAK) 1,2,3 inhibitors have demonstrated efficacy in immune-mediated inflammatory diseases but are also associated with increased CV risk [3, 4]. A therapy for SLE that controls disease activity while providing CV protection is needed. Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor with a unique mechanism of action distinct from JAK 1,2,3 inhibitors. Deucravacitinib has shown clinical efficacy in the 48-week, double-blind, placebo-controlled, phase 2 PAISLEY study in patients with active SLE (NCT03252587).
Objectives: Here, we retrospectively assessed baseline CV risk-associated biomarker profiles and the impact of deucravacitinib treatment on these biomarkers in patients with active SLE from the PAISLEY study.
Methods: Patients were randomized 1:1:1:1 to placebo (n = 90) or deucravacitinib 3 mg twice daily (BID; n = 91), 6 mg BID (n = 93), or 12 mg once daily (n = 89). Plasma samples were collected at baseline and weeks 12, 32, and 48 from 351 patients from the PAISLEY study with available biomarker data and 54 demographically matched normal healthy volunteers (NHVs) to assess protein expression in patients with active SLE vs a healthy reference group and evaluate the impact of deucravacitinib on normalization of the expression levels of key proteins in patients with SLE at week 48. Samples were analyzed via Olink ® Target 96 CVII and CVIII biomarker panels and Olink ® Explore HT, which covers > 5000 proteins. Patients treated in the PAISLEY study were categorized by the presence or absence of known CV disease , including autoimmune comorbidities such as pericarditis, or CV risk factors , primarily hypertension (HTN), based on medical history; these terms are collectively referred to as CV disease herein. Median circulating levels of the identified CV risk-associated proteins in patients with SLE and NHVs were compared using a pairwise Wilcoxon rank sum test. Pharmacodynamic changes in the CV risk-associated biomarkers that were identified in patients with SLE with or without a history of CV disease were evaluated using a linear mixed-effects model. Covariates included age, baseline body mass index, geographic region, baseline glucocorticoid use (< 10 mg/day vs ≥ 10 mg/day), and baseline SLE Disease Activity Index 2000 (SLEDAI-2K) score (< 10 vs ≥ 10).
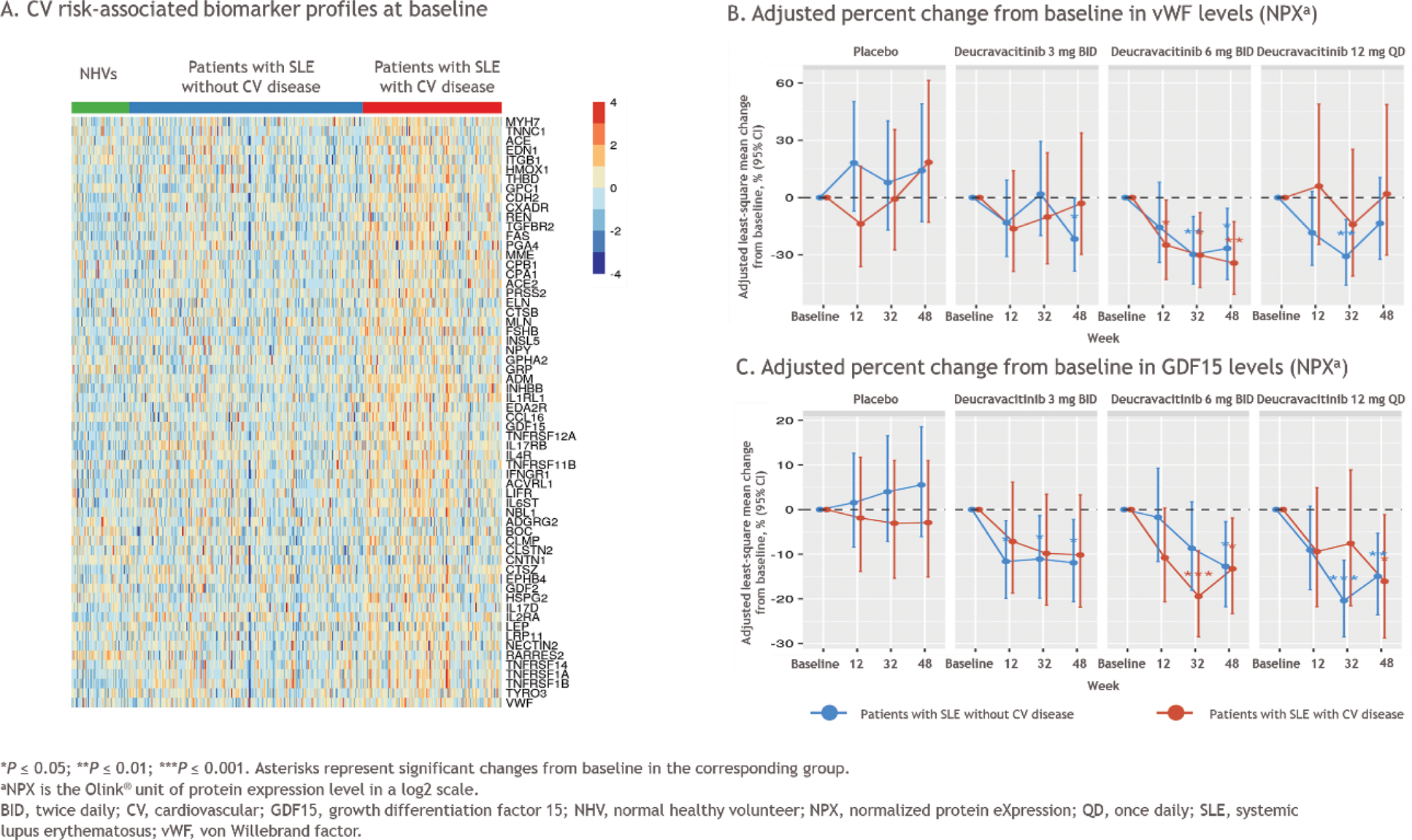
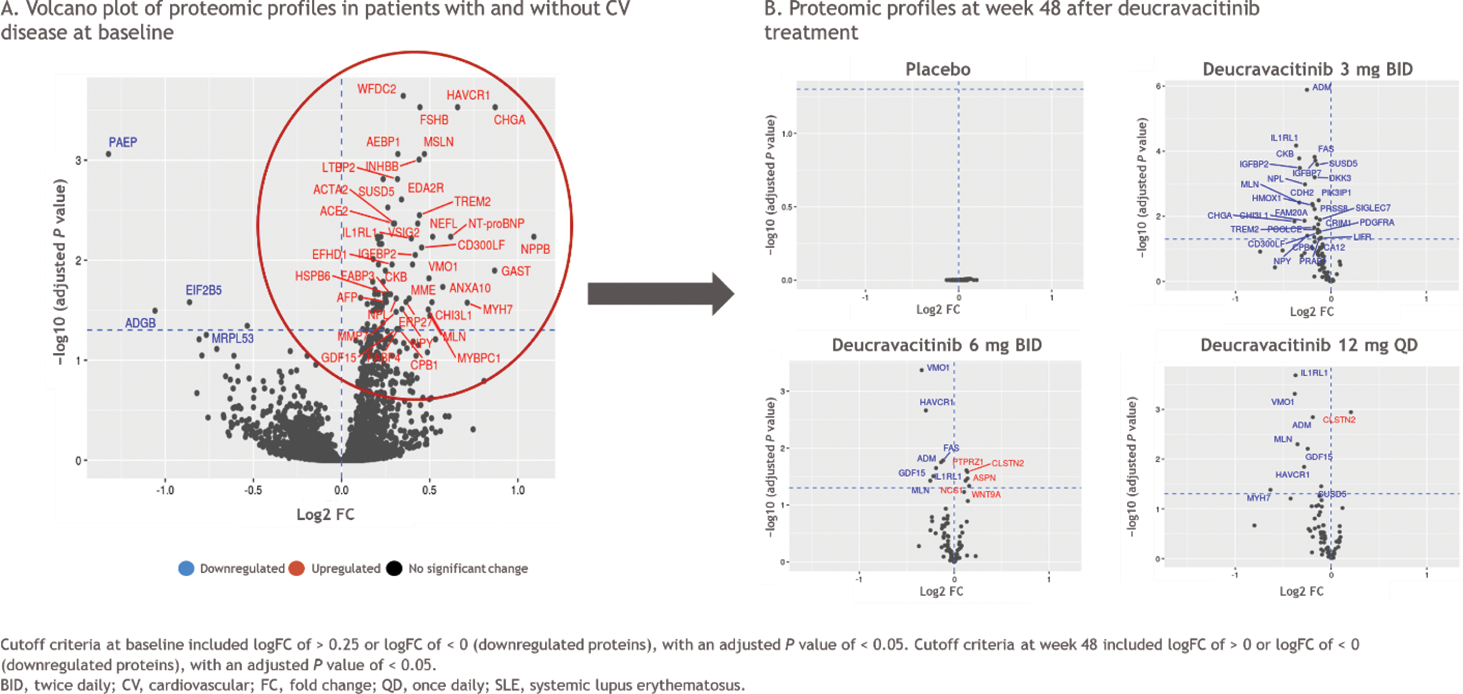
Results: Within the PAISLEY study, 162 patients had CV disease (63 with a known CV disease plus 99 with HTN) and 189 patients did not have a history of CV disease. At baseline, patients with SLE had increased levels of several CV risk-associated biomarkers, including von Willebrand factor (vWF) and growth differentiation factor 15 (GDF15), compared with the NHV reference group; the highest levels were observed in patients with a history of CV disease (Figure 1A). A variable pattern of decreased vWF and GDF15 levels from baseline over time was observed in patients with a history of CV disease who were treated with deucravacitinib (Figure 1B). In those without a history of CV disease, vWF and GDF15 levels decreased from baseline with deucravacitinib treatment by week 48. Patients who received placebo did not have significant changes in vWF or GDF15 levels over time. A differential baseline proteomic profile was identified in patients with SLE with vs without a history of CV disease (Figure 2A). Treatment with deucravacitinib normalized or improved the majority of these proteins, while no meaningful changes were observed with placebo (Figure 2B).
Conclusion: Patients with SLE had elevated CV risk-associated biomarkers at baseline compared with NHVs, and this effect was most apparent in those with a known history of CV disease. Baseline proteomic profiles also differed in patients with vs without a history of CV disease. Treatment with deucravacitinib resulted in improvements in some CV risk-associated biomarkers from baseline in the overall population of patients with SLE and in patients with a history of CV disease, while treatment with placebo did not result in changes in biomarker levels from baseline. Taken together, these results suggest that deucravacitinib may normalize some CV risk-associated biomarkers in patients with active SLE. Further investigation of these findings is warranted in a larger population of patients with SLE from the ongoing phase 3 POETYK SLE-1 (NCT05617677) and POETYK SLE-2 (NCT05620407) studies.
REFERENCES: [1] Yazdany J, et al. RMD Open 2020;6:e001247.
[2] Bello N, et al. Lupus 2023;32:325–341.
[3] Maqsood MH, et al. ACR Open Rheumatol 2022;4:912–922.
[4] Charles-Schoeman C, et al. Ann Rheum Dis 2023;82:119–129.
Acknowledgements: We thank the patients and families who made this study possible, as well as the clinical teams that participated. The study was supported by Bristol Myers Squibb. All authors contributed to and approved the abstract; professional medical writing and editorial assistance was provided by Stephanie V. Koebele, PhD, of Nucleus Global, funded by Bristol Myers Squibb.
Disclosure of Interests: Brittany Weber Consultancy: Bristol Myers Squibb, Novo Nordisk, and Kiniksa Pharmaceuticals, Christina Charles-Schoeman Consultant: Octapharma, Boehringer Ingelheim, Recludix, Sana Biotechnology, Pfizer, AbbVie, Galapagos, and Bristol Myers Squibb, Bristol Myers Squibb, Priovant, CSL Behring, Janssen, Pfizer, and AbbVie, Lu Gao Bristol Myers Squibb, Bristol Myers Squibb, Eric Morand AstraZeneca, Biogen, Bristol Myers Squibb, Dragonfly, EMD Serono, Genentech, Gilead, GSK, Novartis, RemeGen, Takeda, and Zenas, AbbVie, Amgen, AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Genentech, GSK, Janssen, Novartis, Takeda, and UCB, J. Michelle Kahlenberg AstraZeneca, Eli Lilly, GSK, Biogen, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Ventus Therapeutics, Exo Therapeutics, Danger Bio, and Boehringer Ingelheim, Q32 Bio, Celgene/Bristol Myers Squibb, Ventus Therapeutics, Rome Therapeutics, and Janssen, Michael Garshick AGEPHA Pharma, Bristol Myers Squibb, and Kiniksa Pharmaceuticals. Research grants: Pfizer, Julie Zrubek Bristol Myers Squibb, Bristol Myers Squibb, Ian Catlett Employee and shareholder at the time of study conduct: Bristol Myers Squibb, Employee and shareholder at the time of study conduct: Bristol Myers Squibb, Peter Schafer Bristol Myers Squibb, Bristol Myers Squibb, Xueer Chen Bristol Myers Squibb, Bristol Myers Squibb, Chun Wu Bristol Myers Squibb, Bristol Myers Squibb, Jinqi Liu Bristol Myers Squibb, Bristol Myers Squibb.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (