

Background: The randomized, double-blind, double-dummy, active-controlled, Phase 3 ADVOCATE trial investigated whether a treatment regimen including avacopan could replace a standard prednisone taper regimen for patients with granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA), two types of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) [1]. Cyclophosphamide (CYC) remains an option in the standard of care for induction of remission for AAV [2, 3].
Objectives: Conduct a subgroup analysis of the efficacy and safety of avacopan for patients with GPA/MPA who received CYC in the ADVOCATE trial.
Methods: Patients in the ADVOCATE trial received, at investigators’ discretion, either CYC followed by azathioprine (or mycophenolate mofetil if azathioprine was not tolerated) or rituximab. Patients were randomized 1:1 to receive avacopan (30 mg twice daily) or a prednisone taper (60 mg/day tapered to 0 by week 21). This study reports on the outcomes of patients in the CYC subgroup. CYC was administered orally at 2 mg/kg/day (maximum 200 mg/day) for 14 weeks beginning on day 1 or intravenously at 15 mg/kg (maximum 1.2 g) on day 1 and at weeks 2, 4, 7, 10, and 13. Starting at week 15, patients in the CYC subgroup received oral azathioprine at a starting dose of 1 mg/kg/day, with titration up to a target dose of 2 mg/kg/day at 2 weeks, or mycophenolate to a target dose of 2 g/day. Patients with an eGFR <15 mL/minute/1.73 m 2 at screening were excluded from the trial. Key efficacy endpoints were the proportions of patients achieving disease remission at week 26 and sustained remission at week 52. Additional outcomes included relapse, estimated glomerular filtration rate (eGFR), urinary albumin:creatinine ratio (UACR), Glucocorticoid Toxicity Index (GTI), glucocorticoid (GC) dose (prednisone-equivalent), health-related quality of life (HRQoL, as measured by EQ-5D-5L and 36-Item Short Form Health Survey), and safety.
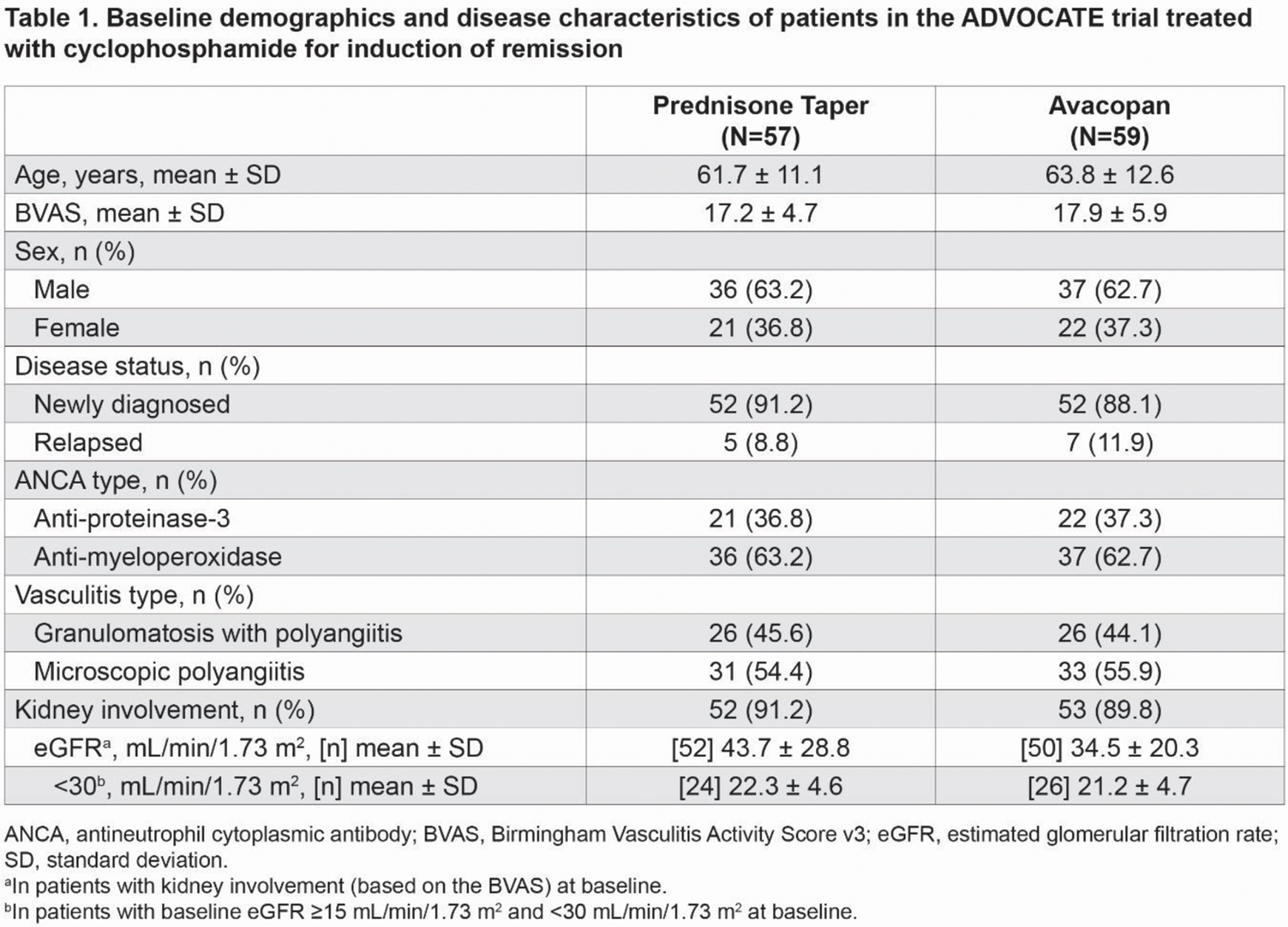
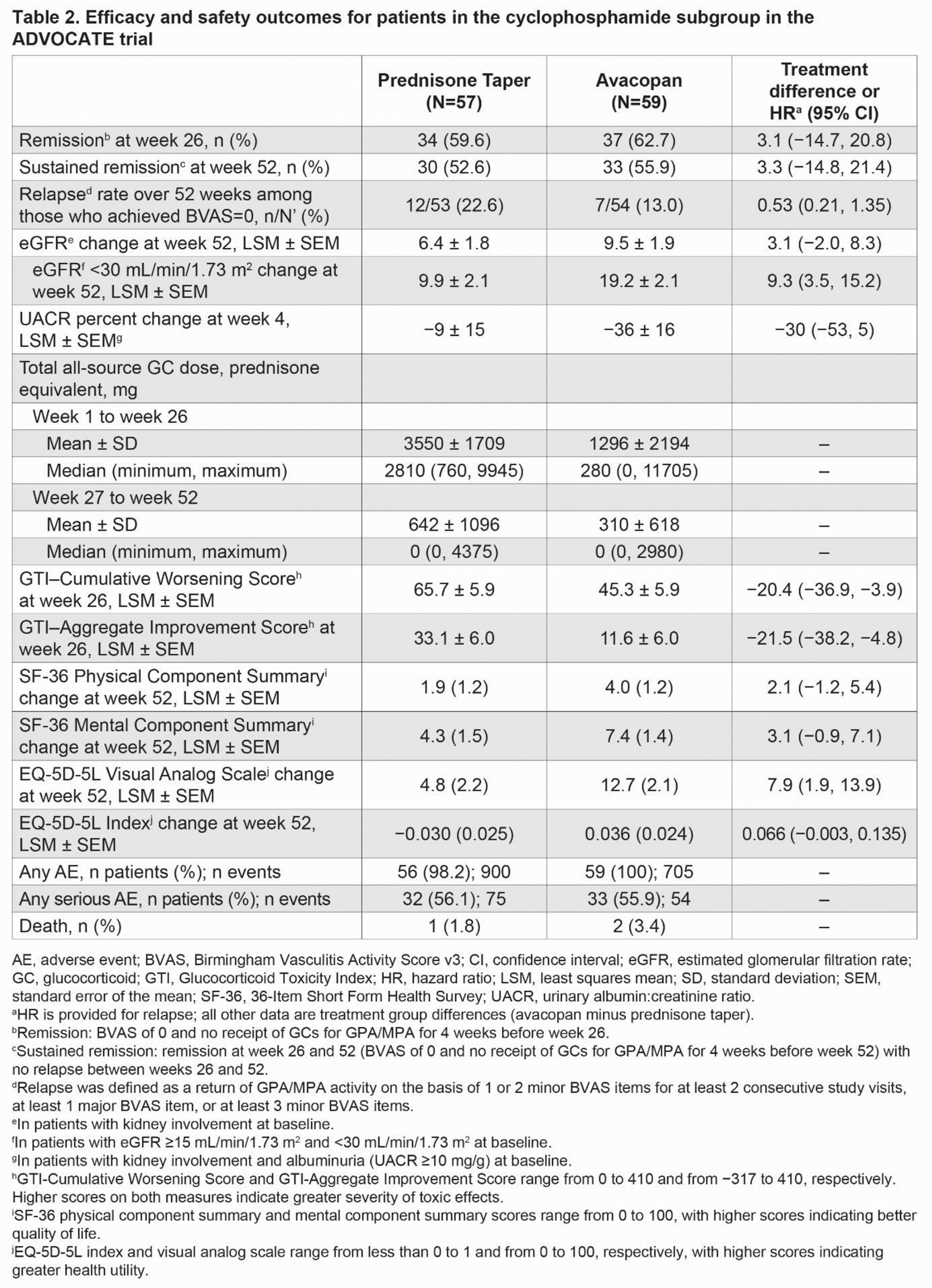
Results: A total of 116 of 330 patients (35.2%) comprised the CYC subgroup in ADVOCATE. At baseline, these patients were 63 years old (mean), 62.9% were male, 90.5% had kidney involvement (based on the Birmingham Vasculitis Activity Score v3 [BVAS]), 62.9% were myeloperoxidase-ANCA positive, and 89.7% were newly diagnosed (Table 1). In patients with kidney involvement at baseline, eGFR (mean ± SD) was 34.5 ± 20.3 mL/min/1.73 m 2 and 43.7 ± 28.8 mL/min/1.73 m 2 for the avacopan and prednisone taper groups, respectively. In patients with a baseline eGFR <30 mL/min/1.73 m 2 , baseline eGFR was similar between the avacopan and prednisone taper groups. The total all-source GC dose (mean ± SD) was 1296 ± 2194 mg for the avacopan group vs 3550 ± 1709 mg for the prednisone taper group from week 1 to week 26, which decreased to 310 ± 618 mg and 642 ± 1096 mg, respectively, from week 27 to week 52 (Table 2). A similar proportion of patients in the avacopan vs prednisone taper groups achieved remission at week 26 (62.7% vs 59.6%; difference: 3.1%; 95% confidence interval [CI]: −14.7%, 20.8%) and sustained remission at week 52 (55.9% vs 52.6%; difference: 3.3%; 95% CI: −14.8%, 21.4%). The relapse rate over 52 weeks among those who achieved BVAS=0 at any time was 13.0% in the avacopan group vs 22.6% in the prednisone taper group (hazard ratio: 0.53; 95% CI: 0.21, 1.35). There were numerically greater improvements from baseline in all measures of HRQoL in the avacopan group vs the prednisone taper group at week 52 (Table 2). In patients with kidney involvement and a baseline eGFR <30 mL/min/1.73 m 2 (n=50), the least-squares mean (LSM) change from baseline in eGFR was 19.2 mL/min/1.73 m 2 in the avacopan group and 9.9 mL/min/1.73 m 2 in the prednisone taper group at week 52 (difference: 9.3; 95% CI: 3.5, 15.2). In patients with kidney involvement and a UACR ≥10 mg/g at baseline, the LSM change from baseline in UACR was −36% in the avacopan group vs −9% in the prednisone taper group at week 4 (difference: −30; 95% CI: −53, 5). The avacopan group had less GC toxicity than the prednisone taper group per the GTI scores at week 26 (Table 2). Serious adverse events occurred in 33 patients (55.9%; 54 events) in the avacopan group and in 32 patients (56.1%; 75 events) in the prednisone taper group. Two (3.4%) deaths occurred in the avacopan group (pneumonia and worsening of vasculitis), and 1 (1.8%) occurred in the prednisone taper group (infectious pleural effusion).
Conclusion: The analysis of the subgroup of patients who received CYC followed by azathioprine (or mycophenolate mofetil) in the ADVOCATE trial suggests similar rates of remission in the avacopan group compared with the prednisone taper group. Use of avacopan with a markedly reduced GC regimen was associated with a numerically lower relapse rate, greater improvement in eGFR, faster reduction in UACR, lower GC-related toxicity, and greater improvements in HRQoL vs a prednisone taper. Safety appeared similar between the two treatment groups. These results support the use of avacopan in combination with CYC for the treatment of GPA/MPA.
REFERENCES: [1] Jayne DRW, et al. N Engl J Med . 2021;384:599-609.
[2] Rajasekaran A, Rizk DV. Kidney360 . 2023;4:1794-1805.
[3] Hellmich B, et al. Ann Rheum Dis. 2024;83:30-47.
Acknowledgements: This study was funded by Amgen Inc. Writing and editorial support was funded by Amgen Inc. and provided by Rachel Gurlin, PhD, of Amgen Inc. and Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company.
Disclosure of Interests: Duvuru Geetha Amgen, Calliditas, GSK, Sana Biotechnology, Vera Therapeutics – Consultant, Thomas Neumann GlaxoSmithKline AG, AstraZeneca GmbH, Amgen Switzerland AG, Vifor Pharma Switzerland – Consultant, Alexandre Karras Vifor, GSK, Otsuka, Astra-Zeneca – Speaker’s bureau, Vifor, GSK, Novartis – Consultant, Maria C. Cid GSK – Paid instructor, GSK, CSL-Vifor – Speaker’s bureau, Royalties from UpToDate – Shareholder, CSL-Vifor, GSK, AstraZeneca, Alexion, Boehringer-Ingelheim, AbbVie, Novartis, Royalty Pharma – Consultant, Kiniksa Pharmaceuticals Ldt – Grant/research support, Peter A Merkel Royalties from UpToDate, Q32 (Stock options or bond holdings in a for-profit corporation or self-directed pension plan), Sparrow (Stock options or bond holdings in a for-profit corporation or self-directed pension plan) – Shareholder, Boehringer Ingelheim – Employee, AbbVie/Abbott, Amgen, argenx, AstraZeneca, Bristol Myers Squibb, Cabaletta, ChemoCentryx, CSL Behring, Dynacure, EMDSerano, Forbius, Genentech/Roche, Genzyme/Sanofi, GSK, HiBio, Immagene, InflaRx, Jannsen, Kiniksa, Kyverna, Magenta, MiroBio, Neutrolis, Novartis, NS Pharma, Pfizer, Q32, Regeneron, Sanofi, Sparrow, Takeda, Talaris, Visterra – Consultant, AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, ChemoCentryx, Eicos, Electra, Forbius, Genentech/Roche, Genzyme/Sanofi, GSK, InflaRx, Sanofi, Takeda, Talaris – Grant/research support, Sarah Bray Amgen – Shareholder, Amgen – Employee, Alana M. Bozeman Amgen, AstraZeneca – Employee, David R. W. Jayne Amgen, GSK, CSL Vifor – Speaker’s bureau, Alentis, Aurinia – Shareholder, AstraZeneca, Boehringer Ingelheim, Chinook, CSL Vifor, GSK, Novartis, Roche – Consultant, GSK, Roche, CSL Vifor – Grant/research support.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (