

Background: Current management guidelines for rheumatoid arthritis (RA) recommend a treat-to-target (T2T) approach, where treatment is adjusted if the goal of remission or low activity is not achieved. Under this approach, disease activity is regularly assessed using a disease activity index, such as the disease activity score (DAS44), which requires a physical examination. However, with the growing demands on our healthcare system, regular physical assessment of our patients may not always be feasible. One of the solutions might be the implementation of patient reported outcome measures (PROMs) to remotely monitor the disease activity. Previous research, in which several dichotomized PROMs were combined, showed acceptable diagnostic accuracy (area under the receiver-operating curve (AUC-ROC) of 0.76) in differentiating between maintaining well-controlled or developing active disease over a 3-monthly period [1]. However, these individual PROMs might also contain items that are not directly associated with disease activity, hindering optimal discrimination. Additionally, time burden to fill out questionnaires should also be taken into account.
Objectives: Therefore, our aim is to develop a model composed of a selection of independent PROM-items, that accurately represents the disease activity score in patients with RA.
Methods: Data were used from early and established RA patients who participated in the tREACH or TARA trial, respectively. Both studies were multicentre, single blinded clinical trials with a treat-to-target design. Treatment alterations were based on the DAS44, measured every 3 months alongside PROMs over 5 years for early RA patients and 2 years for established RA patients. Patients were included if they met the 1987 or 2010 criteria and had at least one PROM-item and DAS44 measurement at the same time point. The data were randomly split (1:1) into development and internal validation cohorts. For external validation, data were used from all RA patients from the Leiden Early Arthritis Clinic (EAC) cohort who were included between 2006 and 2023. This population-based inception cohort treated early RA patients according to current (inter)national guidelines. DAS44 and PROMs were collected biannually during the first year and annually thereafter. Included patients had a medium (interquartile range) follow-up of 3 (1-7) years. The model was developed using a penalised linear regression method (“Least Absolute Shrinkage and Selection Operator” (LASSO)). LASSO can select the most relevant variables from a set of potential predictor variables and decreases overfitting, resulting in a more accurate and generalizable model. Potential predictor variables for the DAS44 value included age, sex, disease duration and all individual PROM items from the Health Assessment Questionnaire (HAQ, 20 items), pain (numeric rating scale 0-10), General Health (GH, visual analogue scale (VAS) 0-100 mm) and fatigue (VAS 0-100 mm). The final PRO-based disease activity score was internally and externally evaluated for its ability to detect active disease (DAS>2.4) using the AUC-ROC. Sensitivity and specificity where then assessed across thresholds based on: (I) the Youden index, which maximizes the sensitivity and specificity; (II) the DAS44 threshold for active disease (DAS44>2.4); and thresholds corresponding to a (III) sensitivity and (IV) specificity of 95% in the development cohort.
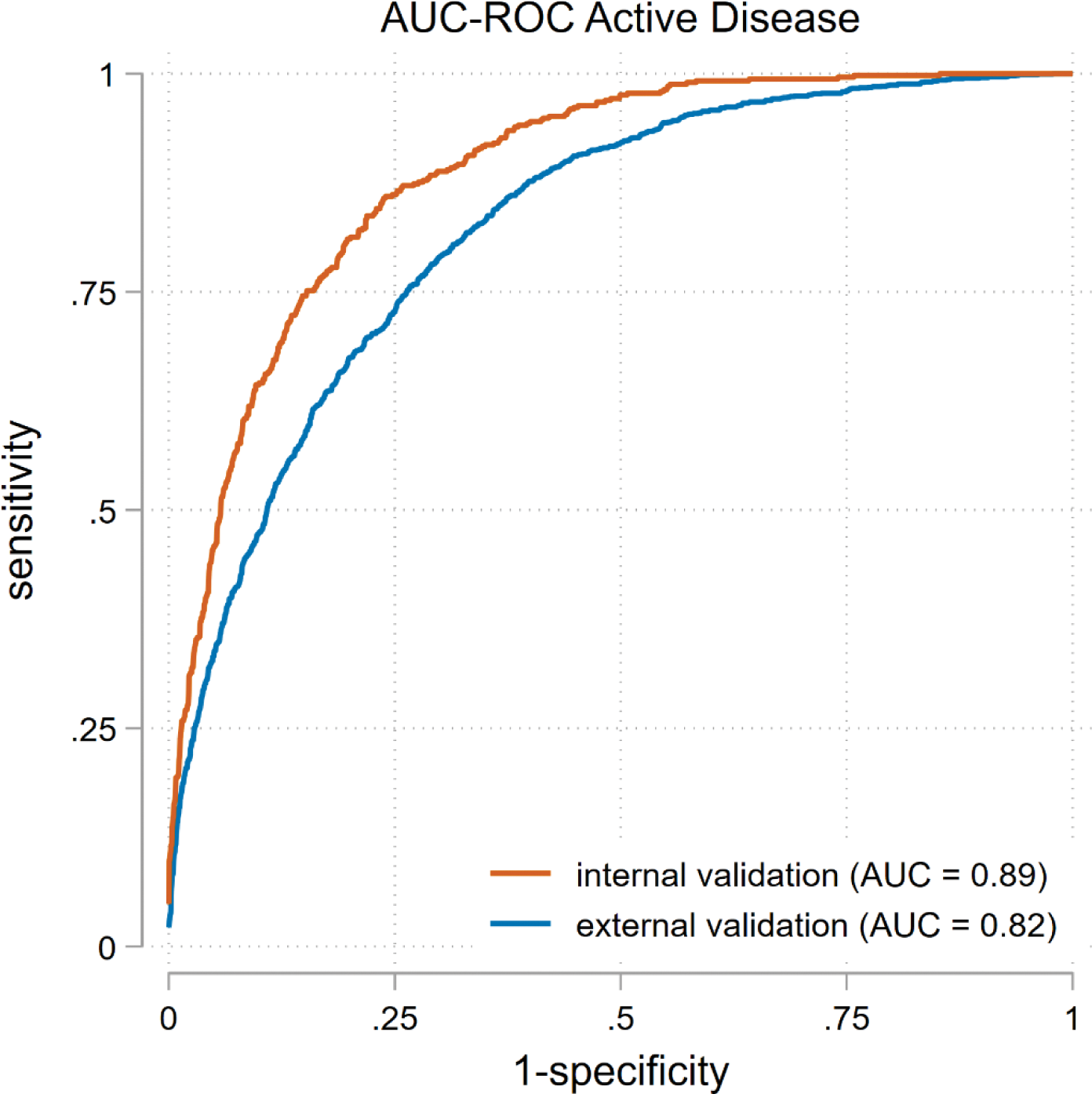
Results: In the development and internal validation cohort, 423 early and 187 established RA patients were included, totaling 5802 visits. Active disease was observed in 1140 (20%) of these visits. The external validation cohort included 793 RA patients with 2467 visits. Active disease was present in 1639 (38%) of these visits. The final model, based on the best-fitting LASSO regression, included 12 of the 26 items: age, sex, disease duration, 7 HAQ-items, pain and GH. The model had an excellent discriminative ability for detecting active disease with an AUC of 0.89 in the internal validation cohort and 0.82 in the external validation cohort. Table 1 summarizes the sensitivity and specificity for the different thresholds. If the Youden index (1.93) is used, the sensitivity for detecting active disease is 78% and the specificity is 82%.
Conclusion: A combination of age, sex, disease duration, VAS GH, NRS pain and 7 items of the HAQ is able to adequately differentiate between well-controlled and active disease with excellent diagnostic accuracy, even after validation in an external dataset. This could support remote monitoring of RA patients.
REFERENCES: [1] Looijen AEM, Snoeck Henkemans SVJ, Van Der Helm-Van Mil AHM, Welsing PMJ, Koc GH, Luime JJ, et al. Combining patient-reported outcome measures to screen for active disease in rheumatoid arthritis and psoriatic arthritis. RMD Open. 2024;10(4):e004687.
Receiver operating characteristic curve for detecting active disease in the internally and externally validated PRO-based disease activity score. Abbreviations AUC, area under the curve; RA, rheumatoid arthritis.
Sensitivity and specificity for different thresholds of the PRO-based disease activity score.
| Threshold 1 | Sensitivity 2 | Specificity 2 | |
|---|---|---|---|
| Youden index | 1.93 | 78 | 82 |
| DAS44 threshold | 2.40 | 52 | 94 |
| 95% sens 1 | 1.40 | 97 | 53 |
| 95% spec 1 | 2.43 | 49 | 94 |
1 Defined in the development cohort
2 Defined in the internal validation cohort
Abbreviations: DAS, disease activity index; Sens, sensitivity; Spec, specificity
Acknowledgements: NIL.
Disclosure of Interests: Agnes E.M. Looijen: None declared, Paco M.J. Welsing: None declared, Sytske Anne Bergstra speeker fees for Benecke, received an ASPIRE grant, Annette H.M. van der Helm – van Mil: None declared, Pascal H.P. de Jong: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (