

Background: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and idiopathic pulmonary fibrosis (IPF) are both associated with the MUC5B promoter variant (rs35705950). Whether these diseases share genetic risk factors beyond this variant is unknown. The publication of genome wide association studies (GWAS) in IPF have facilitated the calculation of polygenic risk scores (PRS) that aggregate genetic risk for IPF across the genome.
Objectives: Investigate the association of an IPF PRS – computed with and without the MUC5B promoter variant – with RA-ILD.
Methods: We identified RA-ILD cases and RA-no-ILD controls from two large biobanks with available genotyping data: the Mass General Brigham Biobank and the UK Biobank. RA-ILD cases met classification criteria for definite or probable RA-ILD in the MGB Biobank [1] and had at least two ICD codes for ILD occurring after RA diagnosis in the UK Biobank. In the MGB Biobank, RA-ILD cases were further subtyped using CT chest imaging findings. RA-noILD comparators had no clinical or imaging evidence of RA-ILD in the MGB Biobank and no administrative codes for ILD in the UK Biobank. In both biobanks, we used available genotyping data and calculated a previously published IPF PRS that excludes the 500,000 bases surrounding the MUC5B promoter variant (IPF no-MUC5B PRS) and also calculated a weighted score that combines this IPF PRS with the MUC5B promoter variant genotype (combined IPF PRS) [2,3]. We examined the associations of the IPF no-MUC5B PRS and combined IPF PRS (continuous score and quintile) with RA-ILD variant using multivariable logistic regression adjusted for age at biobank enrollment, sex, and the first five principal components of genetic ancestry.
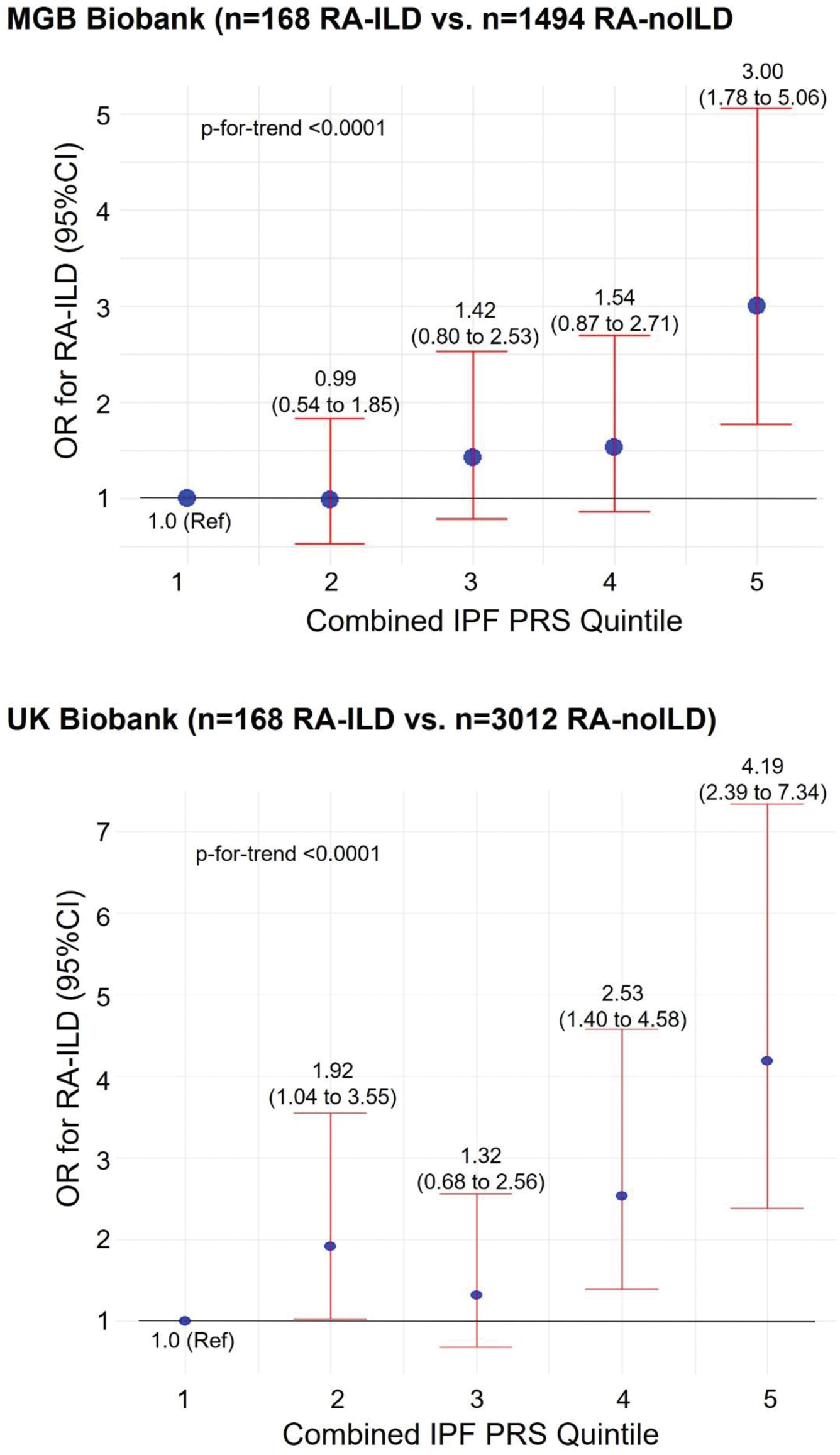
Results: We identified 168 RA-ILD cases and 1494 RA-noILD comparators in the MGB Biobank (mean age= 64.7 vs. 56.3 years and 67% vs 79% females in cases vs. comparators, respectively) and 168 RA-ILD cases and 3012 RA-noILD comparators in the UK Biobank (mean age= 61.8 vs. 59.8 years and 58% vs 79% females in cases vs. comparators respectively). The IPF no-MUC5B PRS, which excluded the MUC5B promoter variant region, was associated with RA-ILD in both the MGB Biobank (OR 1.27, 95%CI 1.05 to 1.55 per standard deviation increase) and UK Biobank (OR 1.52, 95%CI 1.29 to 1.80 per standard deviation increase) (Table 1). The combined IPF PRS, which incorporated the IPF no-MUC5B PRS and the MUC5B genotype in a weighted score, was also associated with RA-ILD (OR 2.35, 95%CI 1.75 to 3.17) as well as with RA-ILD fibrotic subtypes in the MGB Biobank (OR= 3.49, 95%CI 2.12 to 5.76 for RA-ILD with usual interstitial pneumonia subtype) and with RA-ILD in the UK Biobank (OR 2.62, 95%CI 1.95 to 3.51) (Table 1). Among RA patients, the highest quintile of combined IPF PRS had an OR of 3.00 (95%CI 1.78 to 5.06) for RA-ILD in the MGB Biobank and 4.19 (95%CI 2.39 to 7.34) in the UK Biobank (Figure 1). The p-for-trend was <0.0001 across the quintiles of combined IPF PRS in both datasets.
Conclusion: An IPF PRS that excludes the MUC5B promoter variant region was associated with RA-ILD in both the MGB Biobank and UK Biobank. This suggests that IPF and RA-ILD share genetic risk factors beyond the MUC5B promoter variant and PRS may aid risk stratification for RA-ILD.
REFERENCES: [1] Bongartz, Arthritis and Rheumatology, 2010.
[2] Moll, Am J Resp Crit Care Med, 2023.
[3] Allen, Am J Resp Crit Care Med, 2020.
Associations of the MUC5B promoter variant (rs35705950) and idiopathic pulmonary fibrosis polygenic risk score (IPF PRS) with RA-ILD in MGB Biobank and UK Biobank.
| Multivariable* Adjusted OR for RA-ILD in MGB Biobank (95%CI) | Multivariable* Adjusted OR for RA-ILD in UK Biobank (95%CI) | |
|---|---|---|
| Exposure: IPF no-MUC5B PRS (per SD ) | ||
| RA-ILD | 1.27 (1.05 to 1.55 ) | 1.52 (1.29 to 1.80 ) |
| RA-UIP | 1.17 (0.86 to 1.59) | n/a† |
| RA-fibrotic ILD | 1.29 (1.01 to 1.64 ) | n/a† |
| RA-nonfibrotic ILD | 1.27 (0.93 to 1.74) | n/a† |
| Exposure: MUC5B promoter variant (per T allele ) | ||
| RA-ILD | 2.97 (2.16 to 4.10 ) | 2.02 (1.50 to 2.70 ) |
| RA-UIP | 5.17 (3.40 to 7.87 ) | n/a† |
| RA-fibrotic ILD | 4.20 (2.89 to 6.10 ) | n/a† |
| RA-nonfibrotic ILD | 1.30 (0.70 to 2.39) | n/a† |
| Exposure: combined IPF PRS (IPF no-MUC5B + MUC5B promoter variant genotype) (per SD ) | ||
| RA-ILD | 2.35 (1.75 to 3.17 ) | 2.62 (1.95 to 3.51 ) |
| RA-UIP | 3.49 (2.12 to 5.76 ) | n/a† |
| RA-fibrotic ILD | 3.39 (2.19 to 5.26 ) | n/a† |
| RA-nonfibrotic ILD | 1.62 (0.93 to 2.84) | n/a† |
* adjusted for age at enrollment, sex, and the first five principal components of genetic ancestry
† subtyping of RA-ILD not available in UK Biobank
ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis, SD = standard deviation, PRS = polygenic risk score, UIP = usual interstitial pneumonia
Multivariable adjusted OR for RA-ILD by quintile of combined IPF PRS.
Acknowledgements: NIL.
Disclosure of Interests: Gregory McDermott: None declared, Xiaosong Wang: None declared, Jing Cui: None declared, Misti L. Paudel: None declared, Pierre-Antoine Juge Boehringer Ingelheim, Novartis, Janssen, Janssen, Philippe Dieudé: None declared, Matthew Moll 2ndMD, TheaHealth, Sitka, Axon Advisors, Verona Pharma, Sanofi, Bayer, Sean Kalra: None declared, Michael Cho Apogee, Bayer, Jeffrey A. Sparks AbbVie, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Johnson & Johnson, MustangBio, Optum, Pfizer, ReCor, Sana, Sobi, UCB, Bristol Myers Squibb, Boehringer Ingelheim, Sonoma Biotherapeutics.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (