

Background: Pediatric antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare organ- and life-threatening autoimmune disease characterized by inflammation of small- to medium-sized blood vessels [1]. Despite the critical need for targeted therapies, clinical trials specifically addressing pediatric AAV remain limited, forcing reliance on treatment protocols extrapolated from adult data [1]. Whereas current treatment regimens have resulted in better patient outcomes, there are significant safety concerns with existing therapies [1]. Glucocorticoid (GC) toxicity and treatment adherence are especially relevant in younger patients and underscore the need for treatments that better control disease activity while improving safety [2, 3]. Approved as a combination treatment for adults with severe active AAV (granulomatosis with polyangiitis [GPA] or microscopic polyangiitis [MPA]), avacopan is becoming part of the adult standard of care [4]. Based on the similarities between adult and pediatric AAV and the efficacy and safety profile of avacopan in adults, combination treatment with avacopan, a C5aR1 antagonist [5], may offer a novel approach for addressing the unmet medical needs of children and adolescents with GPA/MPA [4].
Objectives: Here we describe a phase 3, open-label, single-arm trial in progress designed to evaluate the efficacy, pharmacokinetics, and safety of avacopan when combined with a rituximab (RTX)- or cyclophosphamide (CYC)-containing regimen in pediatric patients with newly diagnosed or relapsing active GPA/MPA (NCT06321601).
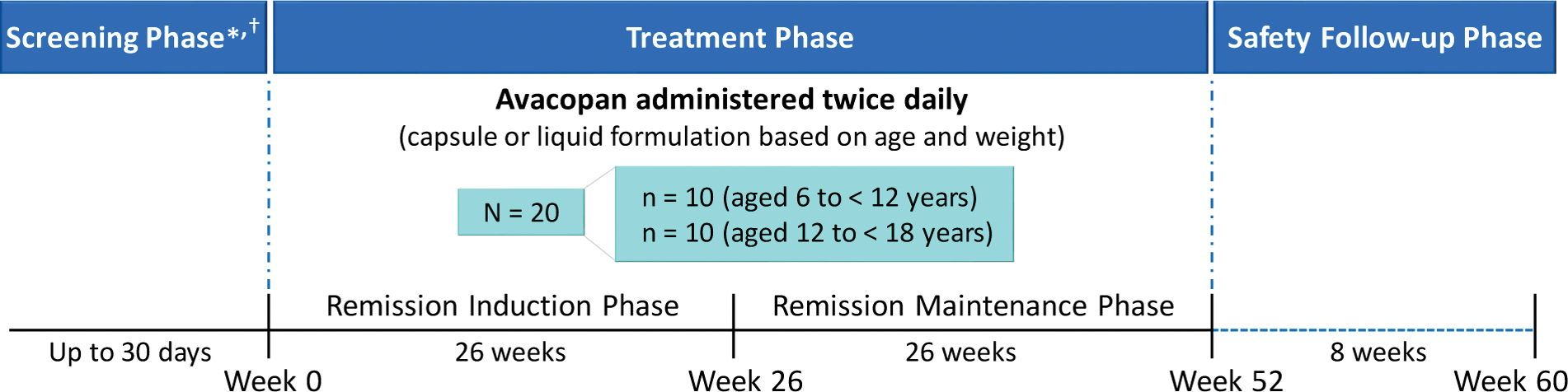
Methods: Target enrollment for the trial is 20 participants (10 patients aged 6 to < 12 years and 10 patients aged 12 to < 18 years; Figure 1). Participants will receive avacopan (with food) twice daily as oral capsules or as a novel liquid formulation, with the dose and formulation based on body weight and age, respectively, for 52 weeks with an 8-week safety follow-up phase. All participants will receive background therapy with an RTX- or CYC-based regimen at the investigator’s discretion. Key inclusion criteria include patients with newly diagnosed or relapsing GPA/MPA positive for anti-proteinase 3 or anti-myeloperoxidase antibodies; active disease with ≥ 1 major item, ≥ 3 nonmajor items, or at least the 2 renal items of proteinuria and hematuria on the Pediatric Vasculitis Activity Score (PVAS); receiving/requiring induction treatment with an RTX- or CYC-based regimen; an estimated glomerular filtration rate (eGFR) of ≥ 15 mL/min/1.73 m 2 ; and a body weight of ≥ 15 kg at day 1. Key exclusion criteria are listed in the Figure 1.
Results: This trial will evaluate the primary endpoints of remission at week 26 (either a PVAS of 0 with a prednisone-equivalent dose of ≤ 0.2 mg/kg/day [maximum dosage of 10 mg/day] within the past 4 weeks or a PVAS of 0 for at least 4 weeks, provided the last PVAS assessment is within the prior 8 weeks, irrespective of the GC dose being received) and sustained remission at week 52 (defined as PVAS-based remission at week 26 and week 52 without relapses [worsening of disease after previously achieving remission involving 1 or 2 PVAS nonmajor items present for 2 consecutive study visits, ≥ 1 PVAS major item, or ≥ 3 PVAS nonmajor items] between the two time points). Key secondary endpoints include plasma concentration measurements of avacopan; safety assessments, including treatment-emergent adverse events; remission by Birmingham Vasculitis Activity Score (BVAS) at week 26 and sustained remission at week 52 (same definitions as stated above with BVAS instead of PVAS); change from baseline in eGFR and urinary albumin to creatinine ratio; Physician Global Assessment; Pediatric Vasculitis Damage Index; cumulative GC dosages administered; and taste and acceptability scores for the liquid formulation (TASTY Faces Scale).
Conclusion: This trial will report on and evaluate the efficacy, safety, and pharmacokinetics of avacopan, administered as either oral capsules or an age-appropriate novel liquid formulation, with an RTX- or CYC-containing regimen in pediatric patients for the treatment of active GPA/MPA. This study will be one of the largest interventional global clinical trials of pediatric patients with active GPA/MPA conducted to date and is expected to yield important insights into the use of avacopan in this patient population.
REFERENCES: [1] Morishita KA, et al. Arthritis Care Res (Hoboken ). 2022;74:1550-1558.
[2] Chen A, et al. Clin Exp Rheumatol . 2022;40:841-848.
[3] Carpenter DM, et al. Clin Rheumatol . 2013;32:649-657.
[4] Zotta F, et al. Pediatr Nephrol . 2023;38:4197-4201.
[5] Jayne DRW, et al. N Engl J Med . 2021;384:599-609.
Trial Schema.*Key inclusion criteria include male and female patients aged 6 to <19 years with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis positive for anti-proteinase 3 or anti-meloperoxidase antibodies; active disease with ≥ 1 major item, ≥ 3 nonmajor items, or at least the 2 renal items of proteinuria and hematuria on the Pediatric Vasculitis Activity Score; receiving/requiring induction treatment with a rituximab- or cyclophosphamide-based regimen; an estimated glomerular filtration rate ≥ 15 mL/mon/1.73 m2; and body weight of ≥ 15 kg at day 1.†Key exclusion criteria include any other known multisystem autoimmune disease including eosinophilic granulomatosis with polyangiitis, alveolar hemorrahage requiring invasive pulmonary ventilation support anticipated to exceed the screening period, and any medical condition requiring or expected to require use of immunosuppressive treatments that may confound study results.
Acknowledgements: This trial is funded by Amgen Inc. Writing and editorial support were funded by Amgen Inc. and provided by Rachel Gurlin, PhD, of Amgen Inc. and Shannon Loftus, PhD, of Red Nucleus.
Disclosure of Interests: Fatima Barbar-Smiley Amgen Inc. (Shareholder), Amgen Inc. (Employee), Eveline Wu Pharming Healthcare Inc. (Speakers bureau), Pharming Healthcare Inc. (Consultant), Sumitomo Pharma Inc. (Consultant), Samuel Gagne: None declared, Pierre Quartier Chugai-Roche (Speakers bureau), Novartis (Speakers bureau), Pfizer (Speakers bureau), Abbvie (Consultant), Amgen Inc. (Consultant), BMS (Consultant), Lilly (Consultant), Novartis (Consultant), Pfizer (Consultant), Sanofi (Consultant), SOBI (Consultant), Amgen Inc. (Grant/research support), BMS (Grant/research support), Lilly (Grant/research support), Novartis (Grant/research support), Pfizer (Grant/research support), Sanofi (Grant/research support), SOBI (Grant/research support), Marinka Twilt: None declared, Thomas Renson Alfasigma (Speakers bureau), Kimberly Morishita: None declared, Zuoshun Zhang Amgen Inc. (Shareholder), Bristol Myers Squibb (Shareholder), Amgen Inc. (Employee), Rajneet K. Oberoi Amgen Inc. (Shareholder), Amgen Inc. (Employee), Hamid Amouzadeh Amgen Inc. (Employee), Rae Yeung: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (