

Background: The presence of MRI subclinical inflammation, in particular hand and wrist tenosynovitis, is associated with future arthritis development in anti-CCP-positive individuals with musculoskeletal (MSK) symptoms [1]. Previous observations suggest tenosynovitis may have a more important role than synovitis in individuals at risk of rheumatoid arthritis (RA). However, this may not be due to their anatomical location, but rather a function of greater total synovial volume (SV) in the tendon sheaths compared with the synovial joints, offering a greater potential for inflammation burden at these sites. Quantitative tenosynovitis and SV in anti-CCP-positive at-risk individuals remain unexplored.
Objectives: To quantitatively assess MRI tenosynovitis volume (TSV) and SV in the hands and wrists of anti-CCP+ at risk individuals and investigate associations (comparative and combined) with future clinical IA development.
Methods: Clinical and demographic data were collected in anti-CCP2 positive individuals with new-onset MSK symptoms without clinical synovitis who were recruited and followed in the Leeds CCP cohort. Baseline contrast-enhanced post-gadolinium 3T MRI scans of the most symptomatic hand were performed, with the images evaluated by a rheumatologist blinded to patient details. The wrist, MCP and PIP joints were assessed for the presence or absence of synovitis, as indicated by contrast-enhancement within the synovium. TSV were calculated by assessment of contrast-enhancing regions along the tendon sheaths of the hands and wrists. Volume calculations were performed by measuring the area of contrast-enhancing regions on axial slices taken at 2-mm intervals, with subsequent 3D reconstruction using OsiriX MD advanced radiological imaging software to ensure precision and continuity. IA progression was defined by the presence of swollen joints during follow-up.
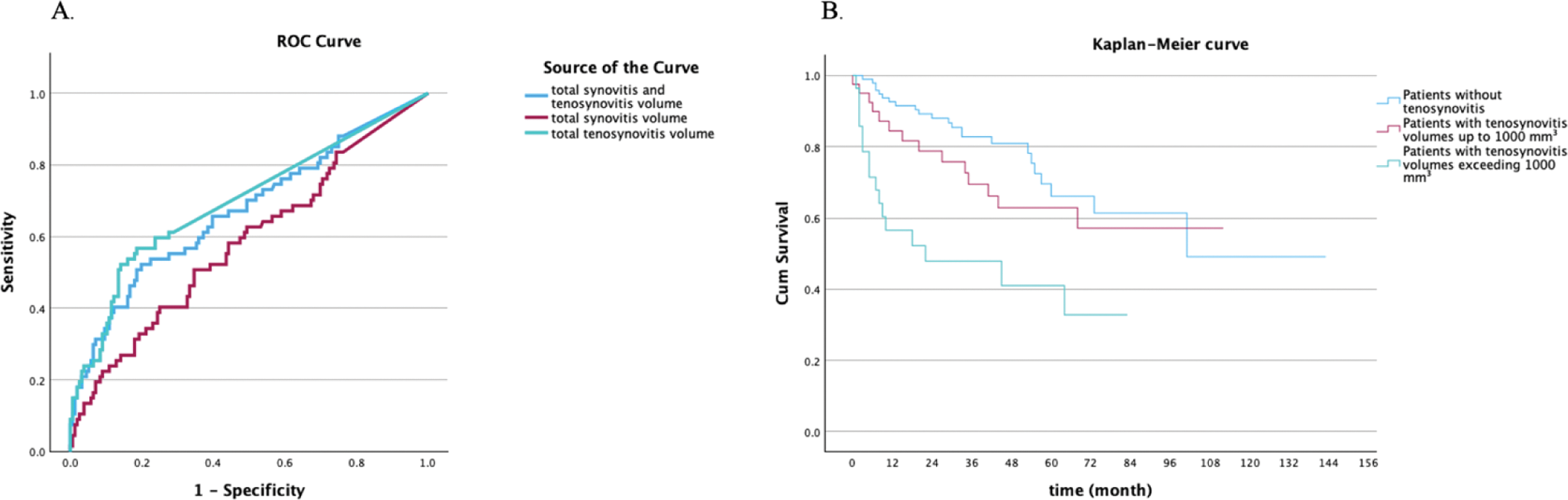
Results: Baseline MRI scans of 223 anti-CCP+ at risk individuals (mean age: 49 ± 13.5 years; 155 female) were analyzed, of whom 67 (30%) progressed to clinical IA over 29.2 (31.9) months. A total of 175 patients (78.5%) had MRI synovitis, with a mean SV of 551 ± 579 mm³. The presence of synovitis showed no significant association with progression to IA. Among patients with synovitis, SV was associated with progression to IA (p=0.04). A total of 77 patients (34.5%) had tenosynovitis, with a mean TSV of 1154 ± 1342 mm³. The presence of tenosynovitis was associated with progression (p<0.001). In those with tenosynovitis, total TSV was associated with progression (p=0.003). Moreover, in patients with either tenosynovitis or synovitis, the combined TSV and SV demonstrated a significantly association with progression (p<0.001) (Table 1). Time to IA progression inversely correlated with combined TSV and SV (r=-0.376, p=0.008). When controlling for TSV, SV showed no significant association with progression (p=0.929); however, TSV remained an independent predictor of progression (r=0.291, p=0.008) when controlling for SV. Additionally, when the effects of combined TSV and SV were controlled for, the ratio of TSV/combined TSV and SV remained significantly associated with progression (p = 0.004). Comparison of areas under the curve (AUC) indicates total TSV demonstrates a stronger predictive ability than the total SV and combined TSV and SV (Figure 1).
Conclusion: In anti-CCP+ at risk individuals without clinical synovitis, MRI tenosynovitis of the hand tendons is associated with a larger synovial tissue inflammatory burden than MRI synovitis. This may be because tenosynovitis is more difficult to detect on clinical examination compared with synovitis. MRI TSV is independently associated with arthritis progression while MRI synovitis is not. Quantitative MRI TSV measurement may therefore be a useful tool for risk stratifying anti-CCP+ at risk individuals and in case selection for preventive interventions.
REFERENCES: [1] van Steenbergen HW, et al. AR 2017;76(3):491-496.
[2] Navalho M, et al. radiology. 2012 (3):823-33.
Comparison of MRI TSV and SV measurements between anti-CCP+ at risk individuals who progressed and did not progress to IA.
| All patients | IA progressors (n=67) | Non-progressors (n=156) | p-value | |
|---|---|---|---|---|
| Age at baseline, mean (%) | 49 (13.5) | 51.1 (13.1) | 48.1 (13.5) | 0.13 |
| Female gender, n (%) | 155 (69.5) | 50 (74.6) | 105 (67.3) | 0.34 |
| Smoking (ever), n (%) | 129 (58.4) | 50 (75.8) | 79 (51) | <0.001 |
| High CCP level*, n (%) | 161 (72.5) | 57 (85.1) | 104 (67.1) | 0.006 |
| Presence of synovitis, n (% ) | 175 (78.5) | 56 (83.6) | 119 (76.3) | 0.29 |
| Total synovitis volume (mm³), mean (SD ) | 551 (579) | 705 (736) | 481 (487) | 0.04 |
| Number of joints with synovitis, mean (SD) | 2.42 (2.48) | 2.45 (2.23) | 2.41 (2.59) | 0.92 |
| Presence of tenosynovitis, n (% ) | 77 (34.5) | 37 (55.2) | 40 (25.6) | <0.001 |
| Total tenosynovitis volume (mm³), mean (SD ) | 1154 (1342) | 1607 (1597) | 726 (864) | 0.003 |
| Number of tendons with tenosynovitis, mean (SD) | 5.6 (3.38) | 5.97 (3.45) | 5.32 (3.34) | 0.41 |
| Total tenosynovitis and synovitis volume (mm³), mean (SD ) | 1.1 (1.4) | 1786 (1876) | 755 (934) | <0.001 |
Analyses comparing TSV and SV were conducted exclusively on patients with tenosynovitis and synovitis, focusing on their respective volumes. Among the wrist stabilizing tendon measurements, the evaluated tendons included the flexor and extensor carpi ulnaris, flexor carpi radialis, abductor pollicis longus, extensor carpi radialis brevis and longus.
* Exceeding three times the ULN
Predictive Performance and Progression -to IA Based on MRI TSV and SV.
1a. ROC curve analysis comparing the predictive performance of total TSV, total SV and combined volumes for IA progression, with AUC values of 0.697, 0.582, and 0.672, respectively. 1b. Kaplan-Meier curve illustrating RA progression stratified into three groups: no tenosynovitis (n = 137), TSV up to 1000 mm³ (n = 53), and TSV exceeding 1000 mm³ (n = 33).
Acknowledgements: NIL.
Disclosure of Interests: Kerem Abacar: None declared, Andrea Di Matteo Janssen, Laurence Duquenne: None declared, Jacqueline Nam: None declared, Paul Emery AbbVie, BMS, Pfizer, MSD, Roche, Janssen, Novartis and UCB, AbbVie, BMS, Pfizer, MSD, Roche, Dennis McGonagle AbbVie, Eli-Lilly, Janssen, Novartis, Pfizer, UCB, AbbVie, Eli-Lilly, Janssen, Novartis, Pfizer, UCB, Kulveer Mankia Abbvie, ALLin Bio, Astra Zeneca, UCB, Lilly, Galapagos, Serac Healthcare, Zura Bio, Deepcure, Gilead, Lilly, Serac Healthcare, Astra Zeneca, Deepcure.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (