

Background: Nonadherence to disease-modifying antirheumatic drugs (DMARDs) is substantial in rheumatoid arthritis (RA) leading to suboptimal clinical outcomes. SQUEEZE is a European Union’s Horizon Europe program-funded project that – among others – aims to develop, implement and evaluate an eHealth-facilitated integrated care model (SCM) to support adherence to DMARDs. Implementing health interventions in general (e.g., clinical recommendations, care models, etc.) is challenging in daily practice. In the SQUEEZE project we performed a contextual analysis (CA) for the implementation of the SCM. A CA examines the environment, conditions, and factors of clinical practice that influence/affect implementation. This analysis helps understand the broader context, allowing for more strategic planning in the design and implementation of health interventions.
Objectives: 1) To unravel practice patterns in RA management; 2) to identify relevant multi-level contextual factors (at the patient, provider, organisation and healthcare system level) in Europe that play a critical role in the development and implementation of the SCM; and 3) to describe the variability in RA management among European countries.
Methods: Following an implementation science approach, the CA was developed based on the components of the Basel Approach for coNtextual ANAlysis (BANANA) [1]: 1) selection of (a) theoretical framework(s); 2) data collection of contextual factors in Europe from the literature and other sources review; 3) involvement of relevant stakeholders, 4) design and conduct of a CA study for a primary data collection of missing CA factors not found in the reviews; 5) determination of the relevance of context for the design and implementation of the SCM; and 6) reporting and dissemination of CA results. Primary data collection (component 4) was performed through structured interviews with RA experts (rheumatologists, nurses, physiotherapists) from 13 European countries: Austria, Belgium, Czech Republic, Denmark, France, Germany, Italy, The Netherlands, Norway, Romania, Spain, Switzerland, United Kingdom.
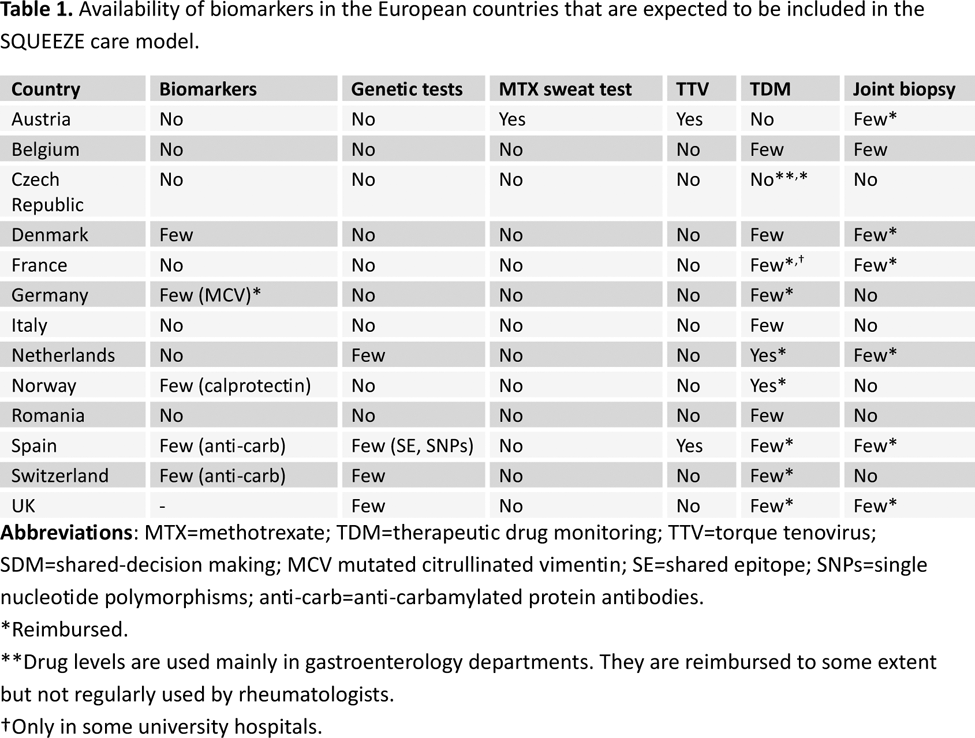
Results: We used the Context and Implementation of Complex Interventions framework to map multi-level contextual factors, and the eHealth Enhanced Chronic Care Model. Key contextual components or factors of the CA (e.g., access to rheumatology care, health workforce, level of digitalization of clinical records, biomarkers availability, etc.) were selected and operationalized using the Chronic Illness Management, Biomedical management and eHealth categories. We present the results of the structured interviews of BANANA component 4. Access to rheumatology services and professionals . We found significant variability in the access and characteristics of health services across European countries. All 13 health systems cover access to rheumatology services, but direct access to rheumatology in the public setting is only possible in 5/13 countries. Although all health systems are inclusive and all patients would be treated in rheumatology if they had RA, there are people with RA who could be at high risk of exclusion, like ethnic minorities, homeless people, low-income families, people in remote rural areas, etc. Continuity of care is not guaranteed in many countries, and it differs across institutions and departments. It may be more likely in second-level rheumatological outpatient clinics and less frequent in university clinics. Access to health care professionals other than rheumatologists is widely varying and, in general, is low. RA related national strategies, programs and registries . Only 6/13 countries reported having a national strategy for RA, and 4/13 programmes targeting specific subgroups of the RA population (e.g., elderly, minorities, pregnancy, etc.). An integrated RA care model, defined as a healthcare approach coordinating various medical services and providers to deliver comprehensive, continuous, and efficient patient care, is available in 8/13 countries. Shared decision-making (SDM ). Connected to clinical practice, SDM principles are apparently applied in most of countries, but there are doubts about its real-life application. The use/number of apps for SDM is in general low. RA SDM tools embedded in the IT system are available in 2/13 countries. IT systems and integration . We also analysed the level of digitalisation of clinical records and access to data, from rheumatology to primary care and vice versa, and of patients to their records. No country reported the highest in all three aspects. Only a small proportion of European countries (3/13) widely have EPROMs embedded in the IT system. In the rest of countries only selected centres. Adherence and patient education . A total of 3/13, 2/13 and 11/13 countries reported the availability of screening and monitoring adherence programs, behavioural or adherence support programmes and educational programs for RA patients, respectively. eHealth . eHealth education for patients and healthcare professionals exists in 6/13 countries. When asked about using apps for therapeutic decisions, the answers were unclear. Only 5/13 countries report eHealth reimbursement programmes. Biomarkers . Finally, regarding the biomarkers that are expected to be included (once validated) in the SCM, most of them are not available in the evaluated countries (see Table 1).
Conclusion: There is a significant variability in the characteristics of health care services across European countries that might impact not only on the SCM development and implementation, but also on the RA management in general.
REFERENCES: [1] DOI: 10.1186/s43058-022-00354-7.
Acknowledgements: The SQUEEZE project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101095052 and from the Swiss State Secretariat for Education, Research and Innovation (SERI).
Disclosure of Interests: Estíbaliz Loza My company (InMusc) has worked as a contracted consultant for laboratories among other institutions, such as BIOHOPE SCIENTIFIC SOLUTIONS FOR HUMAN HEALTH S.L, BMS, Fresenius Kabi, Galápagos, GSK, Lilly, Novo Nordisk Pharma SA, Nordic Pharma, NOVARTIS, Pfizer, Sandoz, SANOFI, Sofia Calado: None declared, Kristina Chingov: None declared, Sabina De Geest: None declared, Agnes Kocher Abbvie, Boehringer Ingelheim, Pfizer, Boehringer Ingelheim, Swiss Nursing Science Foundation, European Union (Horizon Europe) under grant agreement no. 101095052 (SQUEEZE), by UK Research and Innovation (UKRI) under the UK government’s Horizon Europe funding guarantee grant no. 10055567 as well as the Swiss State Secretariat for Education, Research and Innovation (SERI), Teresa Oton My company (InMusc) has worked as a contracted consultant for laboratories among other institutions, such as BIOHOPE SCIENTIFIC SOLUTIONS FOR HUMAN HEALTH S.L, BMS, Fresenius Kabi, Galápagos, GSK, Lilly, Novo Nordisk Pharma SA, Nordic Pharma, NOVARTIS, Pfizer, Sandoz, SANOFI, Janette Ribaut Kantonsspital Winterthur, Berner Fachhochschule, Christina Wettengl: None declared, Loreto Carmona My company (InMusc) has worked as a contracted consultant for laboratories among other institutions, such as BIOHOPE SCIENTIFIC SOLUTIONS FOR HUMAN HEALTH S.L, BMS, Fresenius Kabi, Galápagos, GSK, Lilly, Novo Nordisk Pharma SA, Nordic Pharma, NOVARTIS, Pfizer, Sandoz, SANOFI.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (