

Background: Autologous stem cell transplantation (HSCT) is an evidence-based medical approach to treat systemic sclerosis patients with rapidly progressing disease.
Objectives: To further understand the efficacy of HSCT, the analysis of the process of immune reconstitution after HSCT is elementary. Here, we focused on B cell (memory), namely titers of standard vaccinations against various infectious diseases at different timepoints prior and after HSCT. Besides the titers, we included serum immunoglobulins and the fluorescence-activated cell sorting (FACS) data of CD19+ B cells in our analysis. Consequently, the aim is to investigate the immunological effects particularly in the first two years after HSCT.
Methods: We here show the data of 33 patients transplanted between 2012 and 2021. The tests for different vaccination antibody reactivities were performed at certain time points during HSCT; before transplantation, as well as 6, 12 and 24 months after HSCT. Before transplantation it was recommended to boost missing vaccinations following to the determined titers. Most of the patients showed a sufficient amount of antibodies before the transplantation. To further understand the immune process after an autologous stem cell transplantation, we evaluated the amount of the serum immunoglobulins IgG, IgM and IgA. With our focus on immune reconstitution, we further analyzed the fluorescence-activated cell sorting data of CD19+ B-lymphocytes in our patients.
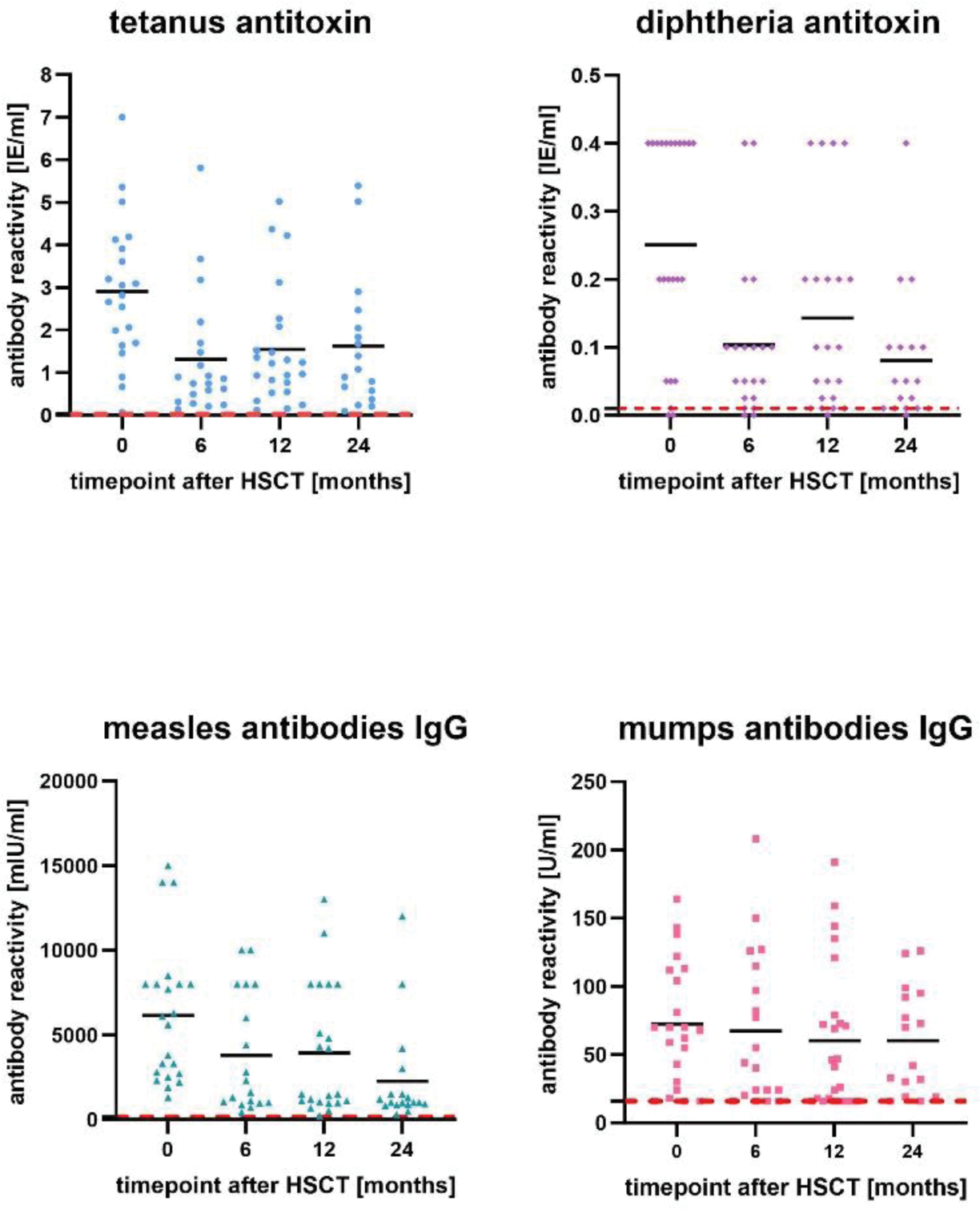
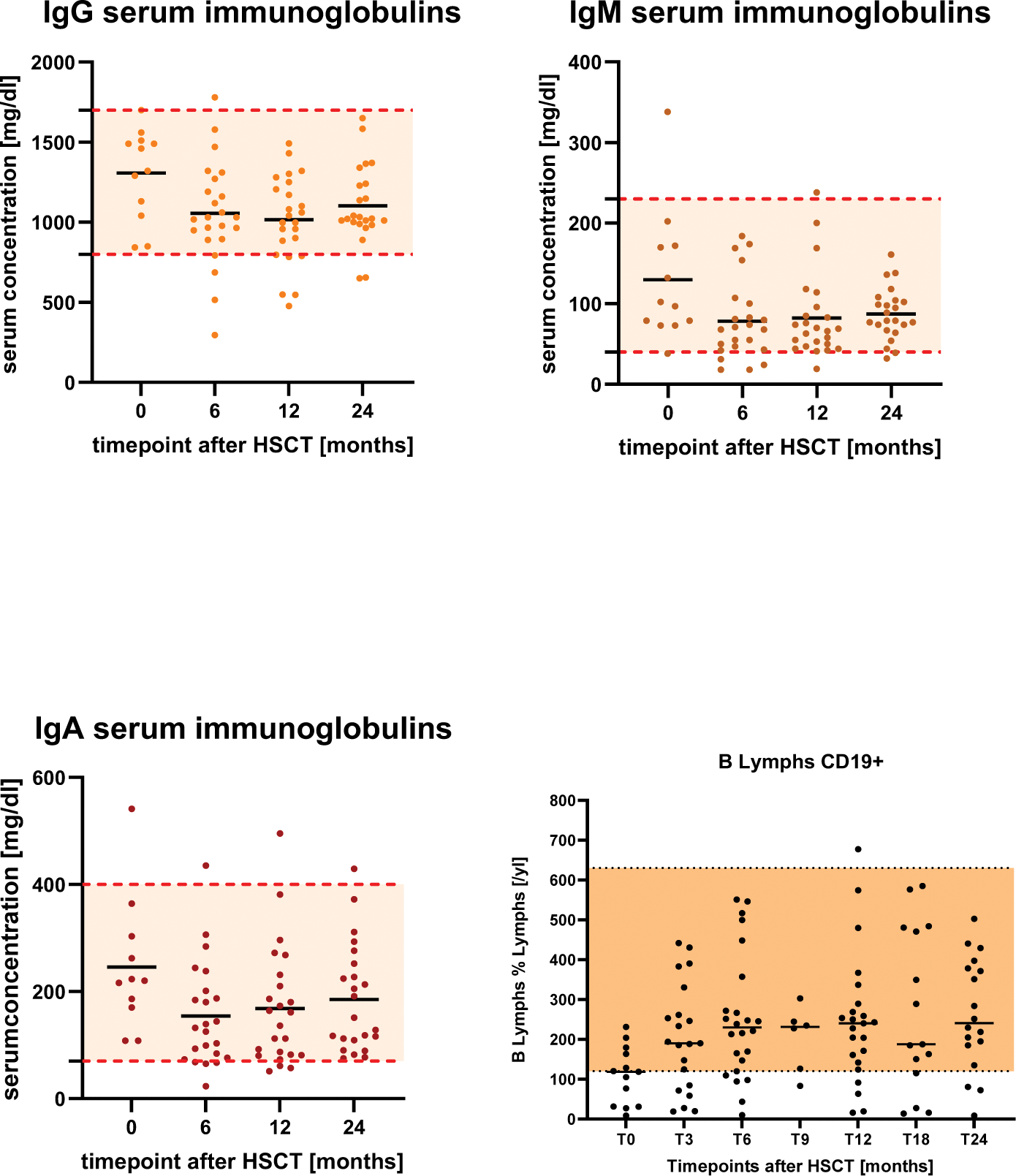
Results: In figure 1, we show the individual values at the different timepoints with the course of the medium value. Moreover, we focused on reference range which most of the patients stayed above during this two-year-time-period. Mainly, a decrease of the titers was seen especially in the first six months after the transplantation. For example, the diphtheria antitoxin titers significantly decreased from month 0 to month 6, t(13)=3.62, p=0.004. Equally the tetanus antitoxin titers t(13)=3.62, p=0.004 and the measles IgG antibodies t(13)=2.84, p=0.015. Some titers significantly decreased within the first year after HSCT which is shown by the mumps IgG antibodies t(16)=2.55, p=0.022. Furthermore, we point out the development of the serum immunoglobulins before and in this two-year-time period after HSCT. (Figure 2) Similarly to the vaccination titers, the IgM serum immunoglobulins decreased in the first 6 months after HSCT t(12)=3.19, p=0.009 as well as IgG serum immunoglobulins in the first 12 months after HSCT t(11)=2.52, p=0.030. Despite the decrease most of the patients stayed on a low to adequate level of protecting titers, recommend by the Standing Committee on Vaccination (STIKO). Besides the investigations of the vaccination titers and serum immunoglobulins, we focused on the numbers of CD19+ B-lymphocytes in our two-year-time period after HSCT. It is shown that most of the patients stayed within the valid reference range. (Figure 2)
Individual values for antibody reactivities (tetanus antitoxin, diphtheria antitoxin, measles antibodies IgG, mumps antibodies IgG) prior and after 6, 12, 24 months after HSCT.
Serum concentrations of IgG, IgM and IgA immunoglobulins and CD19+ B-lymphocytes before and 6,12,24 months after HSCT.
Conclusion: In the majority of the patients a decrease of the vaccination titers and serum immunoglobulins, particularly in the first six to twelve months after HSCT, has been recognized. There might be an assumption that long-living plasma cells in the bone marrow can indicate the humoral immunity. Thus, there is no necessity to blindly boost all patients in this two-year-time period. Monitoring the titers before and after HSCT as well as individually vaccination recommendations are suggested.
Conflict of Interests: Prof. Jörg Henes:
Speakers fee or honoraria from: ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Lilly, Novartis, Pfizer, Roche, SOBI, Otsuka, EUSA, UCB.
AdBoards for: ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Novartis, SOBI, UCB.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Sofie Endres: None declared, Ann-Christin Pecher: None declared, Reinhild Klein: None declared, Jörg Henes ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Lilly, Novartis, Pfizer, Roche, SOBI, Otsuka, EUSA, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (