

Background: Multiple patient-reported outcomes (PROs) have been utilized to capture the overall burden of Systemic Sclerosis (SSc). Specific PROs have been developed and used for overall function/disability (HAQ-DI), hand function (Cochin Hand Function Scale-CHFS), dyspnea (Borg) and gastrointestinal function (University of California Los Angeles Scleroderma Clinical Trials Consortium gastrointestinal tract 2.0-GIT 2.0). This leads to both a multiplication of questionnaire for patients often spending long times to go through partially redundant questions as well as a fragmented picture of how SSc is impacting patients’ lives. The EULAR Systemic Sclerosis Impact of Disease (ScleroID) questionnaire is a comprehensive PRO prioritised by patients and doctors through a validated data driven methodology. The total ScleroID score based on ten domains, Raynaud’s phenomenon, hand function, upper and lower gastrointestinal (GI) tract symptoms, pain, fatigue, body mobility, life choices and activities, digital ulceration, and breathlessness.
Objectives: The aim of the study is to determine the distribution of ScleroID and its domain questions among different disease subsets or disease activity brackets in the context of an observational cohort and to define its sensitivity to change anchored on Minimal clinically important differences (MCIDs) of the other validated PROs to inform the scope of adopting a single comprehensive PRO for clinical trials in SSc.
Methods: Patients with ScleroID questionnaires from the observational cohort STRIKE (Stratification for Risk of Progression in Systemic Sclerosis) were included in the study. Changes (Δ) in scores were calculated as the difference between 12-month follow-up and baseline scores. Spearman’s rank correlation was used to analyse cross-sectional and over-time correlations. MCIDs improvement (i)/worsening (w) of HAQ-DI, CHFS and GIT 2.0 were used as previously described. The Modified version of the EUSTAR Disease Activity Index (mDAI) used for assessing disease activity.
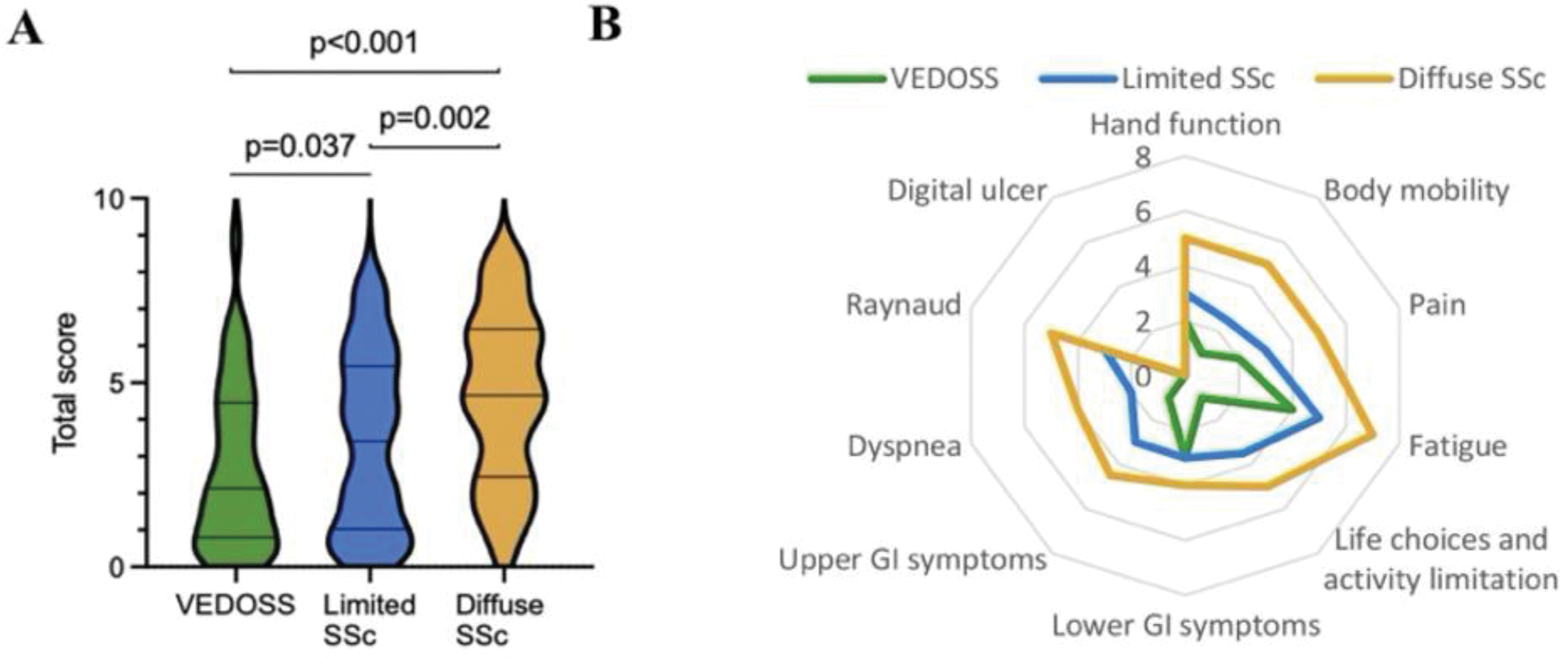
Results: 271 consecutive patients were enrolled between January 2022-2024. Mean age was 54.8±13.9 years. The median (IQR) score of ScleroID was 3.4 (4.2) in all patients, 4.7 (4) in diffuse, 3.4 (4.4) in limited, and 2.1 (3.6) in VEDOSS patients (p<0.05) (Figure 1A). All subitem scores, except lower GI, were higher in diffuse SSc than in VEDOSS patients (p<0.05). Hand function, upper GI, pain, body mobility, dyspnea, and digital ulcer scores were higher in diffuse than limited SSc patients (p<0.05). Limited SSc patients had higher scores of hand function, fatigue, life choices, and dyspnea (p< 0.05) than VEDOSS patients. The radar plot of ScleroID domains clearly shows the growing impact of SSc across the 3 disease subsets (Figure 1B). Patients with PAH had the highest median (IQR) scores for fatigue 8 (6), p=0.04, body mobility 7.5 (7), p=0.02, and dyspnea 6 (5), p<0.001, and patients with ILD had the highest scores for body mobility 5 (5), p=0.007 and dyspnea 4 (5), p<0.001. ScleroID domains and total ScleroID scores showed highly significant correlations with all other PROs (p<0.05), as previously shown, with the strongest correlation for scleroderma HAQ-DI. Patients with high disease activity had higher ScleroID scores than patients with mild activity (median (IQR) scores were 4.8 (4.6) vs 3.7 (4.3), respectively, p=0.003). 12-months of clinical and PRO measures were available for 114 patients. Δ scores of other PROs were correlated with Δ total score and related ScleroID domains with R ranging from 0.211 to 0.486 (p<0.001) (Table 1). Patients who met MCIDi for HAQ-DI had a median (IQR) Δtotal Sclero ID score of 1.2 (4.1), and those who met MCIDw showed -0.6 (3.3) (p<0.001). Similarly, hand function scores were 1 (1.5) in patients who showed MCIDi for CHFS vs -2 (3.5) in patients with MCIDw (p=0.015). Patients who met MCIDi and MCIDw for GIT 2.0 had a median (IQR) Δlower GI 2 (5) and -1 (3) (p<0.001), Δtotal score 0.6 (3.4) and -0.3 (2.1) (p=0.03), respectively.
A. The violin plots of the median and 25-75 th quartiles ScleroID total scores of each disease subset. B . The radar graph of the median scores of each subitems according to disease subsets with the color chosen.
Associations between 12-month changes in clinical measurements and other PROs and ScleroID.
| Δscores | ΔModified Rodnan SS † | ΔBORG | ΔCHFS | ΔUCLA GIT 2.0 | ΔsHAQ |
|---|---|---|---|---|---|
| ΔRaynaud’s | .080 | -.021 | .227** | .132 | .271* |
| ΔHand function | .045 | -.044 | .418* | .116 | .414* |
| ΔUpper Gastrointestinal | -.077 | .012 | .029 | .251* | .128 |
| ΔPain | -.206 | .018 | .284* | .068 | .384* |
| ΔFatigue | .123 | .142 | .166 | .173 | .432* |
| ΔLower Gastrointestinal | -.131 | .008 | -.016 | .392* | .181 |
| ΔLife choices | .012 | .200 | .190 | .325* | .486* |
| ΔBody mobility | -.163 | .144 | .301* | .177 | .480* |
| ΔDyspnea | . -.184 | .334* | -.054 | .125 | .336* |
| ΔDigital ulcer | .042 | .301 | .276 | .192 | .284 |
| ΔTotal score | -.041 | .129 | .258** | .211* | .465* |
Variables given as Spearman’s Rho correlation coefficient. * p <0.01, **p<0.05. Δ presents the difference between follow-up and baseline. mRSS is only given for SSc patients.
Conclusion: ScleroID is a comprehensive, easy to perform PRO able to capture the overall impact of SSc on patients across different disease subsets. ScleroID correlation with validated clinical measures and its sensitivity to change in standard of care setting, support its use as a key composite PRO in clinical practice and clinical trials.
REFERENCES: NIL.
Acknowledgements: None to declare.
Disclosure of Interests: Seda Yurumez Colak: None declared, Stefano Di Donato: None declared, Riccardo Bixio: None declared, Lesley-Anne Bissell: None declared, Theresa Barnes: None declared, Muhammad Nisar: None declared, Vishal Kakkar: None declared, Christopher Denton: None declared, Francesco Del Galdo Astrazeneca, Janssen, Janssen, AstraZeneca, Mitsubishi-Tanabe, AbbVie, AstraZeneca, Janssen, Boehringer-Ingelheim, GSK.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (