

Background: In systemic sclerosis (SSc), the symptoms and signs of small bowel involvement encompass a broad clinical spectrum, ranging from non-specific symptoms such as abdominal pain, distension, nausea, or vomiting to complications such as small intestinal bacterial overgrowth and, in severe cases, intestinal pseudo-obstruction. Currently, manometry is the gold-standard test for small bowel motility evaluation. However, this test is invasive and only available at specialized centers. In recent years, our laboratory has focused on developing innovative non-invasive technologies to evaluate small bowel motor function, focusing on computerized analysis of images obtained by abdominal CT and capsule endoscopy.
Objectives: The aim of this pilot study was to evaluate intestinal motor function in patients with SSc using novel imaging technologies and to compare the results with the gold-standard manometry evaluation.
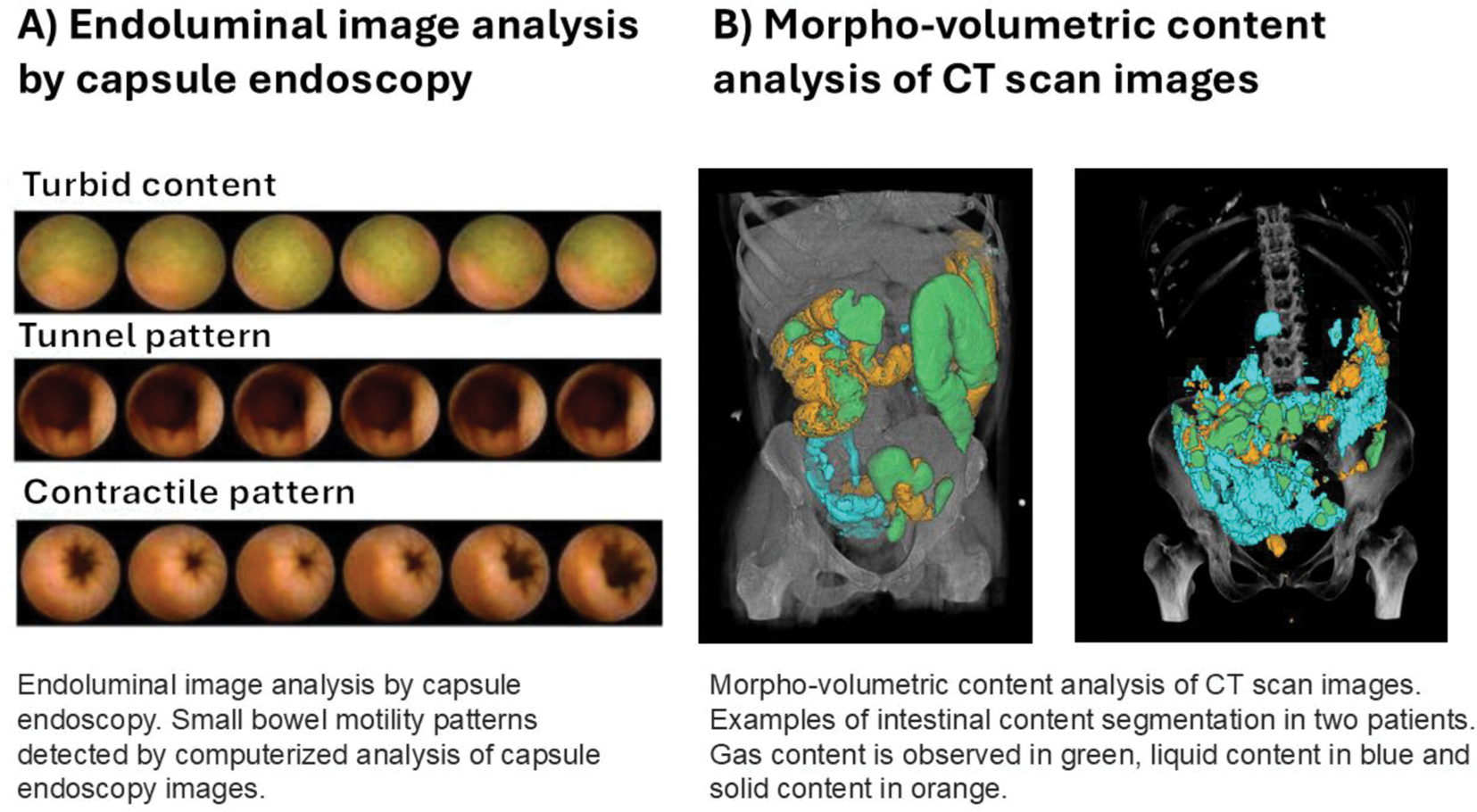
Methods: Prospective study including consecutive patients with SSc and digestive symptoms between June 2021 and June 2024. On separate days, small bowel motility was assessed by high-resolution jejunal manometry, intraluminal images of the small bowel were obtained by capsule endoscopy, and external images by abdominal CT. Jejunal manometry was performed using a 35-channel high-resolution catheter for 5h (3h fasting and 2h postprandial). Tracings were analyzed according to established criteria for small bowel dysmotility and categorized as neuropathic (abnormal contractile activity with preserved amplitude), myopathic (decreased amplitude over the whole tracing), or apparently normal motility. Endoluminal image analysis of capsule endoscopy images was performed using a computer vision program specifically designed to detect contractile patterns (phasic luminal closure and radial wrinkles), non-contractile patterns (tunnel appearance) and turbid-static intestinal content (using color decomposition analysis) (Figure 1A). Morpho-volumetric analysis of CT scan images was performed using a novel end-to-end quasi-automatic framework for image segmentation, which allows quantification of gaseous, liquid, and solid intestinal content (Figure 1B). Morpho-volumetric and endoluminal image analysis results were compared to manometry, stratified by motility patterns.
Results: Seventeen patients with SSc were included in the study (mean age: 54 ± 9 years; 92% female), 6 patients with diffuse cutaneous SSc, and 11 with limited cutaneous SSc. The median disease duration was 12 years (interquartile range: 7–15 years). High-resolution jejunal manometry detected abnormal motility patterns in 11 (61%) patients (5 patients exhibited a neuropathic pattern and 6 a myopathic pattern), and the remaining 6 patients showed apparently normal small bowel motility. Stratification of non-invasive imaging results by high-resolution jejunal manometry patterns revealed that patients with abnormal manometric patterns exhibited increased volumes of intestinal content by CT, as well as an increase in turbid intraluminal content and a reduction in contractile patterns detected by capsule endoscopy, compared to patients with apparently normal motility (Table 1). These alterations were more pronounced in patients with myopathic patterns. Morpho-volumetric analysis of CT scan images and endoluminal image analysis by capsule endoscopy findings showed significant correlations: the volume of liquid intestinal content by CT scan correlated with the presence of turbid intestinal content by capsule endoscopy (r=0.453, p=0.048). Conversely, the volume of solid content on CT scans showed a negative correlation with contractile patterns detected by capsule endoscopy (r-0.624 p=0.007).
Conclusion: Automatic and non-invasive analysis of external and internal images of the gut objectively identifies signs of small bowel dysmotility in patients with SSc, with correlations between the outcomes of both techniques.
REFERENCES: NIL.
| High resolution jejunal manometry results | |||
|---|---|---|---|
| Apparently normal motility (n=6) | Neuropathic patterns (n=5) | Myopathic patterns (n=6) | |
| Morpho-volumetric quantitative analysis (Abdominal CT Scan ) | |||
| Gas content (mL) | 55 (34-73) | 159 (158-190) * | 128 (69-169) * |
| Liquid content (mL) | 58 (51-82) | 176 (138-232) * | 380 (164-535) * |
| Solid content (mL) | 126 (118-169) | 186 (93-200)* | 259 (259-308) * |
| Endoluminal image analysis (capsule endoscopy ) | |||
| Contractile pattern (images/video) | 2644 (2066-3773) | 2212 (1523 – 4999) | 1463 (327 – 3252)* |
| Contractile pattern (%video) | 11 (11-13) | 16 (4-16) | 3 (3-7)* |
| Turbid content (Images/video) | 9077 (2028 – 15090) | 13023 (9856 – 19414)* | 25330 (18684 – 27209)* |
| Turbid content (% video) | 32 (9-41) | 31 (41-51)* | 43 (42-58)* |
| Tunnel pattern (images/video) | 428 (220 – 2013) | 1828 (836 – 1925) | 2850 (1326 – 7188) |
| Tunnel pattern (% video) | 2 (1-2) | 6 (4-6) | 5 (3-5) |
• *P=<0.05 compared to patients with apparently normal motility.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (