

Background: The safety and efficacy of Janus Kinase inhibitors (JAKi) has been broadly studied in clinical trials, and post-authorization observational studies contribute to the knowledge about their safety in routine clinical practice. While JAKi safety is being scrutinized, it is important to explore the safety in specific subgroups of patients and the role of well-known risk factors like sex on the occurrence of adverse events of interest.
Objectives: To explore the potential impact of sex on the incidence of malignancies, infections and cardiovascular events, using real-world data from the JAK-pot collaboration.
Methods: Adult rheumatoid arthritis (RA) patients starting JAKi from 12 registers across Europe and Québec were included. Registers with less than 10 adverse events reported were excluded from the analysis to avoid possible bias. Adverse events of interest were categorized into: all malignancies stratified by “non-melanoma skin cancer (NMSC)” and “malignancy excluding NMSC”, serious infections, major adverse cardiovascular events (MACE) and venous thromboembolism (VTE). Adverse events were attributed to the JAKi treatment if these occurred while on therapy or during a risk window after discontinuation until follow-up loss, death, or study end, whichever came first. The risk windows were 5 years for malignancies, 6 months for cardiovascular events (MACE and VTE), and 3 months for infections. Of note, data from UK were not included for the incidence of malignancies and Canadian were not included for infections due to unavailability/low quality. Incidence rates (IR) per 100 patient-years (PY) and per 1000 PY with 95% confidence intervals (CI) were computed. Poisson regression was used to obtain adjusted incidence rate ratios (aIRR) with 95% CI, accounting for the following covariates: age, disease duration at treatment start, line of treatment, and prior history of each type of event – see Table 2 footnotes.
Results: Of the 65,203 RA patients considered, 12,394 initiated JAKi treatment between 2013 and 2024, and were included for analysis. Patients were mostly females (80.7%), initiated JAKi treatment at a mean age of 57.9 years. The median number of previous b/tsDMARD was 2, thus most treatments courses were a third or later treatment line (21.7%) (Table 1). Overall, patients had similar clinical characteristics, although males seemed to have a later onset of RA and thus a shorter disease duration at treatment start. In addition, males had a higher proportion of overweight/obesity compared to females (67% vs 55.6%), of smokers (54.5% vs 29.8%) and of cardiovascular disease (15.6% vs 7.2%). A total of 286 malignancies (93 NMSC), 1775 infections (319 serious), 69 MACE and 61 VTE were reported. IRs and crude IRRs were lower among females for all events of interest. While the incidence of all malignancies (including NMSC) was significantly lower among female patients (aIRR=0.48 [95% CI: 0.25-0.93]), no differences were found when stratifying by malignancies excluding NMSC, and by NMSC (Table 2). The adjusted incidence rate of serious infections was not significantly different either (aIRR=0.84 [95% CI:0.65-1.08]). Females had a significantly lower incidence of MACE (aIRR=0.39 [95% CI: 0.25-0.63]), yet no significant difference in the incidence of VTE (aIRR=0.60 [95% CI: 0.35-1.04]) was observed between females and males.
Conclusion: In this real-world study, including 12 RA registers with all currently available JAKi, differences were found between males and females in the safety profile: a significantly lower risk of MACE was observed among females compared to males. Results should be interpreted with caution given the limitations of this work, including the unmeasured or residual confounding that could potentially explain some of these findings. Future analyses are planned to further assess sex differences with regards to time from treatment start until the occurrence of the first adverse event, for all outcomes of interest.
REFERENCES: NIL.
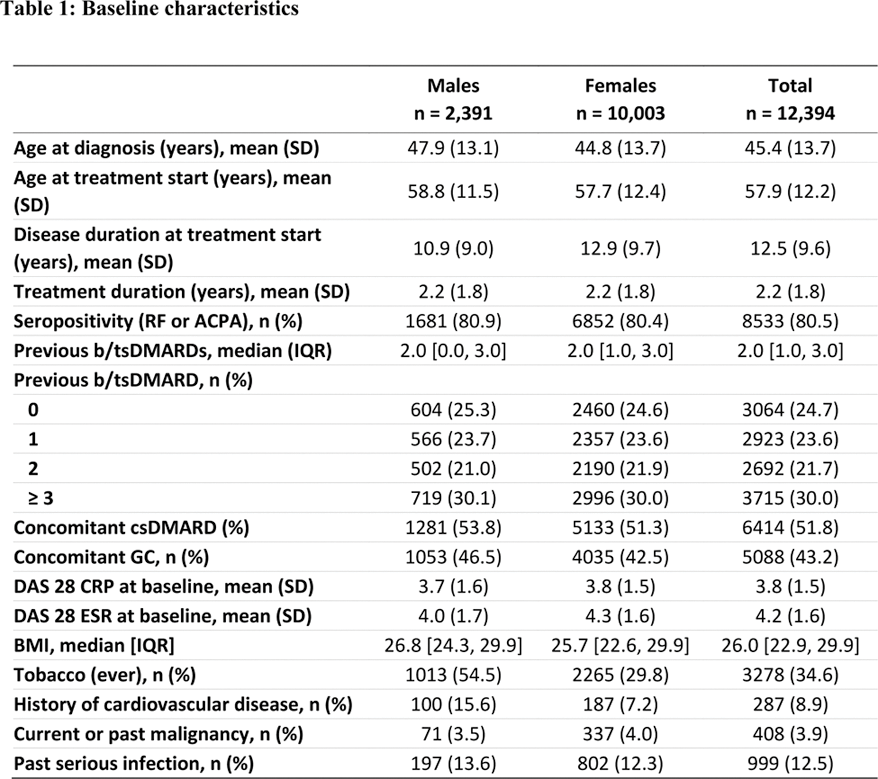
SD = standard deviation, RF = rheumatoid factor, ACPA = anti-citrullinated peptide antibody, b/tsDMARDs = biological or targeted synthetic DMARDs, csDMARDs = conventional synthetic DMARDs, GC = glucocorticoids, DAS 28 = Disease Activity Score 28, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, BMI = Body Mass Index, IQR = interquartile range.
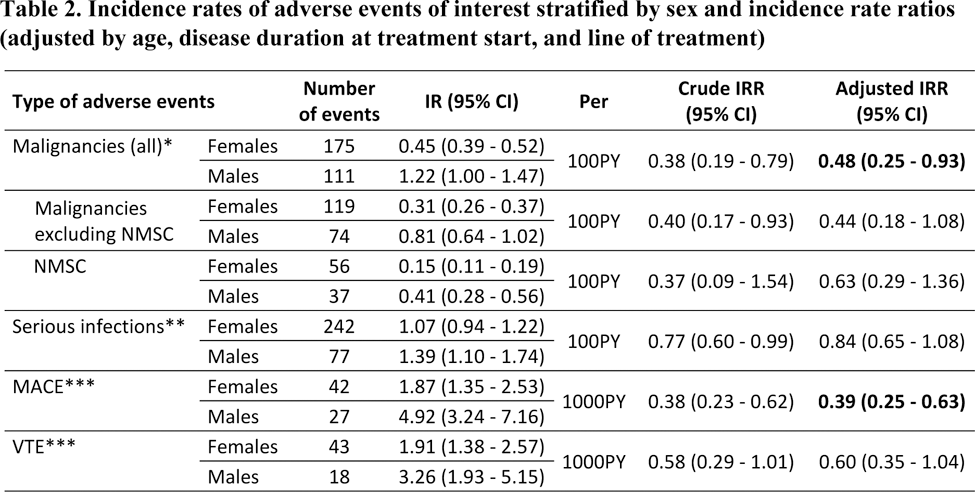
*Additionally adjusted by “current or past malignancy”
**Additionally adjusted by “past serious infection”
***Additionally adjusted by “history of cardiovascular disease”
Acknowledgements: This study is investigator initiated. The JAK-pot collaboration is supported by unconditional/unrestricted research grants from AbbVie Inc., Eli Lilly and Co., and Alfasigma S.p.A., and was previously supported by Pfizer Inc and Galapagos NV.
Disclosure of Interests: Lucía Otero-Varela: None declared, Carlos Sánchez-Piedra: None declared, Romain Aymon: None declared, Denis Mongin: None declared, Benoît Gilbert: None declared, Romain Guemara: None declared, Denis Choquette: None declared, Catalin Codreanu: None declared, Louis Coupal: None declared, Ruth Fritsch-Stork: None declared, Roberto Giacomelli: None declared, Doreen Huschek: None declared, Kimme Hyrich: None declared, Florenzo Iannone Abbvie, Galapagos, Eli-Lilly, Pfizer, UCB, Abbvie, Janssen, UCB, Galapagos, Tore K. Kvien Grünenthal, Janssen, Sandoz, AbbVie, Gilead, Janssen, Novartis, Pfizer, Sandoz, UCB,, AbbVie, BMS, Galapagos, Novartis, Pfizer, UCB, Dan Nordström MSD, Novartis, Pfizer, UCB, MSD, Novartis, Pfizer, UCB, Karel Pavelka AbbVie, Eli Lilly, Sandoz, UCB, Medac, Pfizer, AbbVie, Eli Lilly, Sandoz, UCB, Medac, Pfizer, Manuel Pombo-Suarez: None declared, Sella Aarrestad Provan: None declared, Ziga Rotar Abbvie, Amgen, AstraZeneca, Boehringer, Biogen, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sandoz Lek, Stada, SOBI, Abbvie, AstraZeneca, Boehringer, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sandoz Lek, SOBI, Prodromos Sidiropoulos: None declared, Elsa Vieira-Sousa: None declared, Anja Strangfeld AbbVie, Galapagos, Lilly, Pfizer, Takeda, UCB, Unconditional grant to my institution for the RABBIT register with equal parts from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Galapagos, Hexal, Lilly, MSD, Viatris, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB, Nina Trokovic: None declared, Jakub Závada Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Sanofi, AstraZeneca, Sobi, Abbvie, Novartis, AstraZeneca, Glaxo, Sizheng Steven Zhao: None declared, Delphine S Courvoisier: None declared, Axel Finckh AbbVie, Astra Zeneca, Eli-Lilly, Pfizer, UCB, AbbVie, Alfasigma, Eli-Lilly, Galapagos, Pfizer, Kim Lauper Pfizer.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (