

Background: Autoantibodies (AAbs) against the angiotensin II type 1 receptor (AT 1 R) and the endothelin-1 type A receptor (ET A R) are hypothesized to be an essential element in the pathophysiology of systemic sclerosis (SSc), stimulating vascular dysregulation and inflammation. Studies have shown conflicting data whether these AAbs are associated with clinical outcomes and mortality in SSc.
Objectives: To determine the presence of AAbs against AT 1 R and ET A R in SSc patients, and to find out if these AAbs are associated with more severe disease manifestations and mortality related to SSc.
Methods: Serum samples from n=279 SSc patients from the prospective Leiden Combined Care in Systemic Sclerosis (CCISS) cohort, n=42 patients with primary Raynaud’s phenomenon (PRP), n=24 patients with rheumatoid arthritis (RA) and n=20 healthy controls (HC) were tested for the presence of anti-AT 1 R- and anti-ET A R AAbs by ELISA. Test-retest reliability was calculated using a Spearman’s rank order correlation test for 29 SSc patients. Comparisons between groups were performed with Mann-Whitney U tests or Kruskall Wallis tests, where appropriate. Associations between AAb levels and disease manifestations related to SSc were determined by calculating risk ratios; Kaplan Meier analyses were used to determine associations between AAbs and survival. To corroborate the results, the same analyses were performed in a validation cohort with n=310 SSc patients from the Radboud University Medical Center.
Results: Patients from the Leiden CCISS cohort did not have significantly higher levels of anti-AT 1 R- or anti-ET A R AAbs compared to patients with PRP, RA, or HCs. Only when pooling the patients with PRP, RA, and the HCs into one non-SSc group, and comparing this to the SSc patients, patients with SSc showed significantly higher levels of anti-AT 1 R AAbs (p=0.043), but not for anti-ET A R AAbs. Strong correlations were found between the unit levels of the 29 SSc patients measured twice for both anti-AT 1 R- and anti-ET A R AAbs (R=0.76, p <0.0001 and R=0.82, p <0.0001, respectively). A strong correlation between anti-AT 1 R- and anti-ET A R AAbs was observed (R=0.81, p <0.001). Using the previously defined cut-off [1)] 81% (n=226) of the SSc patients were positive for anti-AT 1 R AAbs, and 73% (n=203) were positive for anti-ET A R Aabs. We also calculated our own cut-off based on the mean level in units plus two times the SD of healthy controls, which resulted in a cut-off of 52.29 units for anti-AT 1 R AAbs and a cut-off of 32.92 units for anti-ET A R AAbs. Using this new cut-off, 9% (n=26) of the SSc patients were positive for anti-AT 1 R AAbs, and 8% (n=21) were positive for anti-ET A R AAbs. SSc patients with interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), scleroderma renal crisis (SRC), diffuse cutaneous SSc (dcSSc), digital ulcers (DU), death, or all pooled vascular manifestations did not have significantly higher levels of anti-AT 1 R- or anti-ET A R AAbs. No significant associations determined by risk ratios based on the cutoffs previously published could be found between the presence of anti-AT 1 R- or anti-ET A R AAbs and disease manifestations or mortality in SSc. In the SSc cohort from the Radboudumc (n=310), again a strong correlation was found between anti-AT 1 R- and anti-ET A R AAb levels (R= 0.80, p <0.001). Here, patients with dcSSc showed significantly higher median anti-ET A R AAb levels compared to SSc patients with a different disease subset (median=8.1 units vs median=5.9 units, p=0.001), and patients with ILD showed significantly higher median anti-ET A R AAb levels compared to SSc patients without ILD (median=7.6 units vs median=6.1 units, p=0.007). There was no significant difference between median levels in other outcome groups, except for mortality, in which statistically significant differences were found for both anti-AT 1 R AAbs or anti-ET A R AAbs and death, with median levels being lower in the patients that died (anti-AT 1 R AAb: median=6.5 units vs median=7.8 units, p=0.005; anti-ET A R AAb: median=4.9 units vs median=7.0 units, p=0.020). This contrasts with previous studies, in which higher AAb levels were associated with mortality.
Conclusion: In this study we could not validate previously shown associations between higher levels of anti-AT 1 R- and anti-ET A R AAbs and disease manifestations in SSc. Based on the current study, the determination of these antibodies is of limited predictive value in everyday clinical practice.
REFERENCES: [1] Riemekasten G, Philippe A, Näther M, Slowinski T, Müller DN, Heidecke H, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530-6.
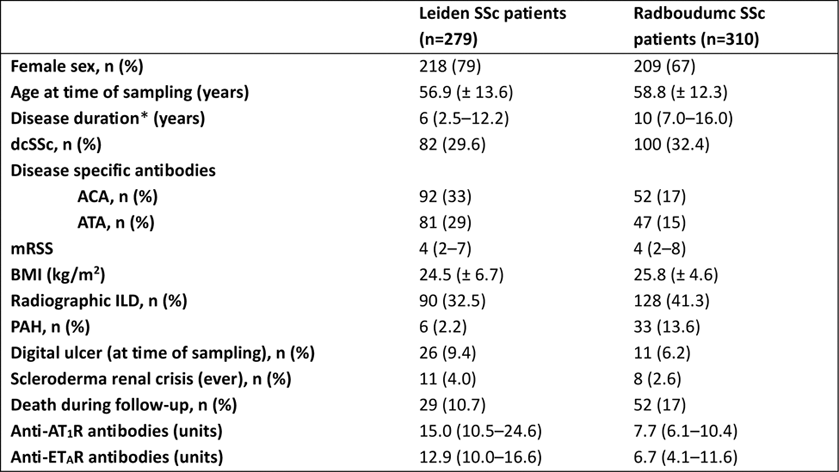
Table 1. Patient characteristics of SSc patients in the Leiden and Nijmegen cohort. Parametric data are reported as mean ± SD, non-parametric data as median (IQR). *Time since first non-Raynaud’s symptom. SSc: systemic sclerosis; dcSSc: diffuse cutaneous systemic sclerosis; ACA: anti-centromere antibodies; ATA: anti-topoisomerase antibodies; mRSS: modified Rodnan skin score; BMI: body mass index; ILD: interstitial lung disease; PAH: pulmonary arterial hypertension; AT 1 R: angiotensin II type 1 receptor; ET A R: endothelin-1 type A receptor.
Association between the occurrence of a clinical outcome and median autoantibody levels in the Leiden SSc cohort. PAH: pulmonary arterial hypertension; ILD: interstitial lung disease; SRC: scleroderma renal crisis; dcSSc: diffuse cutaneous systemic sclerosis; DU: digital ulcers; Vasc. manifest: pooled outcome of all vascular manifestations; AT 1 R: angiotensin II type 1 receptor; ET A R: endothelin-1 type A receptor; AAb: autoantibody.

Acknowledgements: NIL.
Disclosure of Interests: Katherine E. van der Wouden: None declared, Saad Ahmed J&J-Innovative Medicine, J&J-Innovative Medicine, Wieke van Oostveen: None declared, Eva Hoekstra: None declared, Sophie Liem: None declared, Tom Huizinga: None declared, Rene E.M. Toes: None declared, Alexandre E. Voskuyl: None declared, Gabriela Riemekasten: None declared, Cynthia Fehres: None declared, Madelon Vonk Received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, MSD, Novartis, and Roche, Received consulting fees from Boehringer Ingelheim and Janssen Pharmaceutical Companies of Johnson & Johnson, Jeska K. de Vries-Bouwstra Abbvie, Janssen, Boehringer-Ingelheim, Abbvie, Janssen, Boehringer- Ingelheim, Janssen-Cilag, Galapagos, Roche.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (