

Background: Ianalumab, an afucosylated monoclonal antibody, depletes B cells through enhanced antibody-dependent cellular cytotoxicity with concurrent blockade of signals mediated by B-cell–activating factor (BAFF):BAFF receptor (BAFF-R) [1]. It is currently being investigated for the treatment of immune-mediated diseases. Given its novel mechanism of action, it is crucial to assess the effects of ianalumab on preexisting antibodies against vaccine antigens.
Objectives: The study evaluated the impact of ianalumab treatment versus placebo on preexisting antibody levels against 7 pathogens in patients with systemic lupus erythematosus (SLE) and Sjögren’s disease (SjD).
Methods: A retrospective analysis was conducted on serum samples from 2 randomised, double-blind, placebo-controlled phase 2 studies in patients with SjD (NCT02962895) or SLE (NCT03656562). Patients received either placebo or ianalumab 300 mg subcutaneous monthly for 24 weeks (79 patients with SjD) or for 28 weeks (40 patients with SLE). In the SjD study, patients on 300 mg ianalumab at Week 24 (W24) were re-randomised to receive double-blinded monthly ianalumab 300 mg or placebo until W52. Patients on placebo at W24 were switched to a lower ianalumab dose and were not subject to further testing in this analysis. In the SLE study, all patients switched from double-blind to open-label ianalumab up to W52. Antibodies (IgG isotypes) to vaccine antigens and autoantibodies were measured at baseline, W24 (SjD) or W28 (SLE) and W52. Changes in antibody levels from baseline and proportions of patients maintaining protective levels at W52 were assessed. A total of 3 patients (2 SjD and 1 SLE) received booster doses against diphtheria and tetanus toxoid (TTd) under ianalumab treatment.
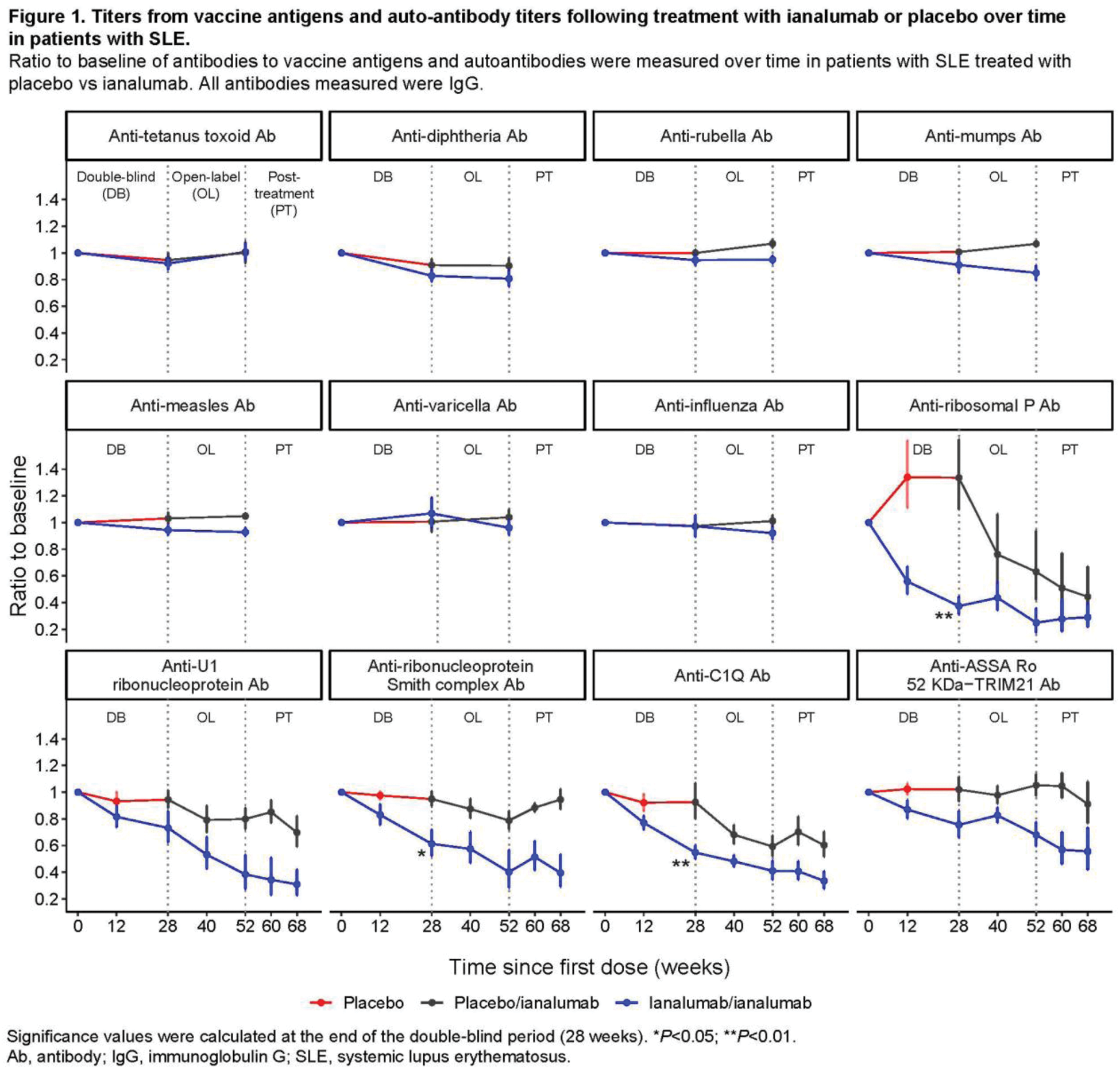
Results: The proportion of patients with SjD and SLE maintaining protective levels of antibodies against TTd, measles, mumps, varicella, rubella, diphtheria and influenza remained stable after ianalumab treatment up to 52 weeks. In patients with SjD, the changes from baseline to W24 were <6% for all antigens in both ianalumab- and placebo-treated patients. In patients with SLE, the changes from baseline to W28 were <10% in both ianalumab- and placebo-treated patients for all antigens besides diphtheria (Figure 1). For diphtheria, up to 18% changes were observed under ianalumab treatment, likely due to the low level of preexisting protection (<50% of patients had protective levels at baseline). In contrast, several autoantibodies showed a significant reduction in ianalumab-treated patients (e.g. up to 60% reduction of anti-ribosomal P antibodies at W28) compared to placebo (Figure 1). These results are in line with the ability of ianalumab to deplete memory and antibody-producing cells [2], while likely not affecting the long-lived bone marrow plasma cells that do not express BAFF-R [3]. Among the 3 patients who received booster dose(s) against diphtheria and TTd during ianalumab treatment, 2 showed a subsequent increase in corresponding titers, whereas the other patient received the booster only 10 days before W52 sampling, likely explaining the lack of increased titers.
Conclusion: Treatment with ianalumab up to 52 weeks did not result in a reduction of the antibody titers to previous immunizations against tetanus, varicella, measles, mumps, rubella, diphtheria and influenza while having a clear impact on autoantibody levels.
REFERENCES: [1] McWilliams EM, et al. Blood Adv 2019;3(3):447–460.
[2] Dörner T, et al. Ann Rheum Dis 2024;83:956–957.
[3] Darce JR, et al. J Immunol 2007;179 (11):7276–7286.
Acknowledgements: Authors thank Alexandre Avrameas for his contribution in generating the auto-Ab data.
Disclosure of Interests: Thomas Dörner Consulting fees from AbbVie, Celgene, Eli Lilly, EMD MerckSerono, GSK, Janssen, Novartis, Roche, and Gilead/Galapagos, Received grants from AbbVie, Celgene, Eli Lilly, EMD MerckSerono, GSK, Janssen, Novartis, Roche, UCB, Sanofi, Deutsche Forschungsgemeinschaft and EU Horizon2020 HarmonicSS, Nan Shen: None declared, Thomas Grader-Beck Received consulting fees from Novartis, Argenx, and BMS, Received grants from Novartis, Argenx, and BMS, Caroline Walter Shareholder of Novartis, Employee of Novartis, Catherine Wioland Shareholder of Novartis, Employee of Novartis, Celine Rauld Shareholder of Novartis, Employee of Novartis, Patrick Schmutz Shareholder of Novartis, Employee of Novartis, Simone Riek Shareholder of Novartis, Employee of Novartis, Wolfgang Hueber Shareholder of Novartis, Employee of Novartis, Carole Sips Shareholder of Novartis, Employee of Novartis, Stephen Oliver Shareholder of Novartis, Employee of Novartis, Carol Lau Shareholder of Novartis, Employee of Novartis, Claire Bonal Shareholder of Novartis, Employee of Novartis, Isabelle Isnardi Shareholder of Novartis, Employee of Novartis.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (